Reporting on the Value of Artificial Intelligence in Predicting the Optimal Embryo for Transfer: A Systematic Review including Data Synthesis
- PMID: 35327499
- PMCID: PMC8945147
- DOI: 10.3390/biomedicines10030697
Reporting on the Value of Artificial Intelligence in Predicting the Optimal Embryo for Transfer: A Systematic Review including Data Synthesis
Abstract
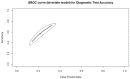
Artificial intelligence (AI) has been gaining support in the field of in vitro fertilization (IVF). Despite the promising existing data, AI cannot yet claim gold-standard status, which serves as the rationale for this study. This systematic review and data synthesis aims to evaluate and report on the predictive capabilities of AI-based prediction models regarding IVF outcome. The study has been registered in PROSPERO (CRD42021242097). Following a systematic search of the literature in Pubmed/Medline, Embase, and Cochrane Central Library, 18 studies were identified as eligible for inclusion. Regarding live-birth, the Area Under the Curve (AUC) of the Summary Receiver Operating Characteristics (SROC) was 0.905, while the partial AUC (pAUC) was 0.755. The Observed: Expected ratio was 1.12 (95%CI: 0.26-2.37; 95%PI: 0.02-6.54). Regarding clinical pregnancy with fetal heartbeat, the AUC of the SROC was 0.722, while the pAUC was 0.774. The O:E ratio was 0.77 (95%CI: 0.54-1.05; 95%PI: 0.21-1.62). According to this data synthesis, the majority of the AI-based prediction models are successful in accurately predicting the IVF outcome regarding live birth, clinical pregnancy, clinical pregnancy with fetal heartbeat, and ploidy status. This review attempted to compare between AI and human prediction capabilities, and although studies do not allow for a meta-analysis, this systematic review indicates that the AI-based prediction models perform rather similarly to the embryologists' evaluations. While AI models appear marginally more effective, they still have some way to go before they can claim to significantly surpass the clinical embryologists' predictive competence.
Keywords: IVF; artificial intelligence; data-synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Embryo selection through artificial intelligence versus embryologists: a systematic review.Hum Reprod Open. 2023 Aug 15;2023(3):hoad031. doi: 10.1093/hropen/hoad031. eCollection 2023. Hum Reprod Open. 2023. PMID: 37588797 Free PMC article.
-
Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF.Hum Reprod. 2020 Apr 28;35(4):770-784. doi: 10.1093/humrep/deaa013. Hum Reprod. 2020. PMID: 32240301 Free PMC article.
-
In vitro fertilization and multiple pregnancies: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(18):1-63. Epub 2006 Oct 1. Ont Health Technol Assess Ser. 2006. PMID: 23074488 Free PMC article.
-
Accuracy of artificial intelligence on histology prediction and detection of colorectal polyps: a systematic review and meta-analysis.Gastrointest Endosc. 2020 Jul;92(1):11-22.e6. doi: 10.1016/j.gie.2020.02.033. Epub 2020 Feb 29. Gastrointest Endosc. 2020. PMID: 32119938
-
What Are the Applications and Limitations of Artificial Intelligence for Fracture Detection and Classification in Orthopaedic Trauma Imaging? A Systematic Review.Clin Orthop Relat Res. 2019 Nov;477(11):2482-2491. doi: 10.1097/CORR.0000000000000848. Clin Orthop Relat Res. 2019. PMID: 31283727 Free PMC article.
Cited by
-
Criteria for implementing artificial intelligence systems in reproductive medicine.Clin Exp Reprod Med. 2024 Mar;51(1):1-12. doi: 10.5653/cerm.2023.06009. Epub 2023 Dec 1. Clin Exp Reprod Med. 2024. PMID: 38035589 Free PMC article.
-
An annotated human blastocyst dataset to benchmark deep learning architectures for in vitro fertilization.Sci Data. 2023 May 11;10(1):271. doi: 10.1038/s41597-023-02182-3. Sci Data. 2023. PMID: 37169791 Free PMC article.
-
Assessment of artificial intelligence model and manual morphokinetic annotation system as embryo grading methods for successful live birth prediction: a retrospective monocentric study.Reprod Biol Endocrinol. 2024 Mar 5;22(1):27. doi: 10.1186/s12958-024-01198-7. Reprod Biol Endocrinol. 2024. PMID: 38443941 Free PMC article.
-
Beyond black-box models: explainable AI for embryo ploidy prediction and patient-centric consultation.J Assist Reprod Genet. 2024 Sep;41(9):2349-2358. doi: 10.1007/s10815-024-03178-7. Epub 2024 Jul 4. J Assist Reprod Genet. 2024. PMID: 38963605 Free PMC article.
-
Application of a methodological framework for the development and multicenter validation of reliable artificial intelligence in embryo evaluation.Reprod Biol Endocrinol. 2025 Jan 31;23(1):16. doi: 10.1186/s12958-025-01351-w. Reprod Biol Endocrinol. 2025. PMID: 39891250 Free PMC article.
References
-
- Murray C.J.L., Callender C.S.K.H., Kulikoff X.R., Srinivasan V., Abate D., Abate K.H., Abay S.M., Abbasi N., Abbastabar H., Abdela J., et al. Population and Fertility by Age and Sex for 195 Countries and Territories, 1950–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1995–2051. doi: 10.1016/S0140-6736(18)32278-5. - DOI - PMC - PubMed
-
- Barash O., Ivani K., Huen N., Willman S., Weckstein L. Morphology of the Blastocysts Is the Single Most Important Factor Affecting Clinical Pregnancy Rates in IVF PGS Cycles with Single Embryo Transfers. Fertil. Steril. 2017;108:e99. doi: 10.1016/j.fertnstert.2017.07.301. - DOI
-
- Chen T.-J., Zheng W.-L., Liu C.-H., Huang I., Lai H.-H., Liu M. Using Deep Learning with Large Dataset of Microscope Images to Develop an Automated Embryo Grading System. FandR. 2019;1:51–56. doi: 10.1142/S2661318219500051. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

