Metformin and Cancer Hallmarks: Molecular Mechanisms in Thyroid, Prostate and Head and Neck Cancer Models
- PMID: 35327549
- PMCID: PMC8945547
- DOI: 10.3390/biom12030357
Metformin and Cancer Hallmarks: Molecular Mechanisms in Thyroid, Prostate and Head and Neck Cancer Models
Abstract
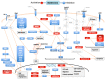
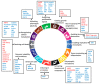
Metformin is the most used drug for type 2 diabetes (T2DM). Its antitumor activity has been described by clinical studies showing reduced risk of cancer development in T2DM patients, as well as management of T2DM compared with those receiving other glucose-lowering drugs. Metformin has a plethora of molecular actions in cancer cells. This review focused on in vitro data on the action mechanisms of metformin on thyroid, prostate and head and neck cancer. AMPK activation regulating specific downstream targets is a constant antineoplastic activity in different types of cancer; however, AMPK-independent mechanisms are also relevant. In vitro evidence makes it clear that depending on the type of tumor, metformin has different actions; its effects may be modulated by different cell conditions (for instance, presence of HPV infection), or it may regulate tissue-specific factors, such as the Na+/I- symporter (NIS) and androgen receptors. The hallmarks of cancer are a set of functional features acquired by the cell during malignant development. In vitro studies show that metformin regulates almost all the hallmarks of cancer. Interestingly, metformin is one of these therapeutic agents with the potential to synergize with other chemotherapeutic agents, with low cost, low side effects and high positive consequences. Some questions are still challenging: Are metformin in vitro data able to translate from bench to bedside? Does metformin affect drug resistance? Can metformin be used as a generic anticancer drug for all types of tumors? Which are the specific actions of metformin on the peculiarities of each type of cancer? Several clinical trials are in progress or have been concluded for repurposing metformin as an anticancer drug. The continuous efforts in the field and future in vitro studies will be essential to corroborate clinical trials results and to elucidate the raised questions.
Keywords: cancer; hallmarks; head and neck; in vitro; mechanisms; metformin; prostate; thyroid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A review for clinicians: Prostate cancer and the antineoplastic properties of metformin.Urol Oncol. 2017 Jan;35(1):21-29. doi: 10.1016/j.urolonc.2016.10.009. Epub 2016 Nov 9. Urol Oncol. 2017. PMID: 27836248 Review.
-
Research Progress on the Use of Metformin in Leukemia Treatment.Curr Treat Options Oncol. 2024 Feb;25(2):220-236. doi: 10.1007/s11864-024-01179-3. Epub 2024 Jan 30. Curr Treat Options Oncol. 2024. PMID: 38286894 Free PMC article. Review.
-
Impact of Metformin and compound C on NIS expression and iodine uptake in vitro and in vivo: a role for CRE in AMPK modulation of thyroid function.Thyroid. 2014 Jan;24(1):78-87. doi: 10.1089/thy.2013.0041. Epub 2013 Sep 25. Thyroid. 2014. PMID: 23819433
-
Metformin as an Anticancer Agent.Trends Pharmacol Sci. 2018 Oct;39(10):867-878. doi: 10.1016/j.tips.2018.07.006. Epub 2018 Aug 24. Trends Pharmacol Sci. 2018. PMID: 30150001 Free PMC article. Review.
-
What every dentist should know about metformin, diabetes, and cancer.Gen Dent. 2015 Sep-Oct;63(5):70-2. Gen Dent. 2015. PMID: 26325646 Review.
Cited by
-
Effect of metformin alone and in combination with etoposide and epirubicin on proliferation, apoptosis, necrosis, and migration of B-CPAP and SW cells as thyroid cancer cell lines.Res Pharm Sci. 2023 Jan 19;18(2):185-201. doi: 10.4103/1735-5362.367797. eCollection 2023 Apr. Res Pharm Sci. 2023. PMID: 36873273 Free PMC article.
-
Antitumor Effects and Mechanisms of Metabolic Syndrome Medications on Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2022 Dec 14;9:1279-1298. doi: 10.2147/JHC.S392051. eCollection 2022. J Hepatocell Carcinoma. 2022. PMID: 36545268 Free PMC article. Review.
-
Drug repositioning in thyroid cancer treatment: the intriguing case of anti-diabetic drugs.Front Pharmacol. 2023 Dec 11;14:1303844. doi: 10.3389/fphar.2023.1303844. eCollection 2023. Front Pharmacol. 2023. PMID: 38146457 Free PMC article. Review.
-
The development and benefits of metformin in various diseases.Front Med. 2023 Jun;17(3):388-431. doi: 10.1007/s11684-023-0998-6. Epub 2023 Jul 4. Front Med. 2023. PMID: 37402952 Review.
-
Metabolomic Profiling of Oral Potentially Malignant Disorders and Its Clinical Values.Biomedicines. 2024 Dec 19;12(12):2899. doi: 10.3390/biomedicines12122899. Biomedicines. 2024. PMID: 39767805 Free PMC article. Review.
References
-
- Jiao Y., Wang X., Luo Z. Preventive and (Neo)Adjuvant Therapeutic Effects of Metformin on Cancer. IntechOpen; London, UK: 2020. pp. 1–22. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

