Novel Therapeutic Target for Prevention of Neurodegenerative Diseases: Modulation of Neuroinflammation with Sig-1R Ligands
- PMID: 35327555
- PMCID: PMC8945408
- DOI: 10.3390/biom12030363
Novel Therapeutic Target for Prevention of Neurodegenerative Diseases: Modulation of Neuroinflammation with Sig-1R Ligands
Abstract
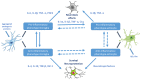
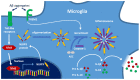
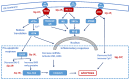
Neurodegenerative diseases (NDDs) are characterized by progressive deterioration of the structure and function of cells and their networks in the nervous system. There are currently no drugs or other treatments that can stop the progression of NDDs. NDDs have many similarities and common pathways, e.g., formation of misfolded amyloid proteins, intra- and extracellular amyloid deposits, and chronic inflammation. Initially, the inflammation process has a cytoprotective function; however, an elevated and prolonged immune response has damaging effects and causes cell death. Neuroinflammation has been a target of drug development for treating and curing NDDs. Treatment of different NDDs with non-steroid anti-inflammatory drugs (NSAIDs) has failed or has given inconsistent results. The use of NSAIDs in diagnosed Alzheimer's disease is currently not recommended. Sigma-1 receptor (Sig-1R) is a novel target for NDD drug development. Sig-1R plays a key role in cellular stress signaling, and it regulates endoplasmic reticulum stress and unfolded protein response. Activation of Sig-1R provides neuroprotection in cell cultures and animal studies. Clinical trials demonstrated that several Sig-1R agonists (pridopidine, ANAVEX3-71, fluvoxamine, dextrometorphan) and their combinations have a neuroprotective effect and slow down the progression of distinct NDDs.
Keywords: Alzheimer’s disease; ER-stress; astrocytes; cytokines; microglia; neurodegenerative diseases; neuroinflammation; non-steroid anti-inflammatory drugs; sigma-1 receptor ligands.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tan S.H., Karri V., Tay N.W.R., Chang K.H., Ah H.Y., Ng P.Q., Ho H.S., Keh H.W., Candasamy M. Emerging pathways to neurodegeneration: Dissecting the critical molecular mechanisms in Alzheimer’s disease, Parkinson’s disease. Biomed. Pharmacother. 2019;111:765–777. doi: 10.1016/j.biopha.2018.12.101. - DOI - PubMed
-
- Nakamura T., Lipton S.A. Neurodegenerative Diseases as Protein Misfolding Disorders. Volume 1 Oxford University Press; Oxford, UK: 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

