Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes
- PMID: 35327565
- PMCID: PMC8945211
- DOI: 10.3390/biom12030373
Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes
Abstract
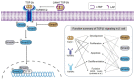
Beta (β) cell dysfunction or loss is the common pathological feature in all types of diabetes mellitus (diabetes). Resolving the underlying mechanism may facilitate the treatment of diabetes by preserving the β cell population and function. It is known that TGF-β signaling plays diverse roles in β cell development, function, proliferation, apoptosis, and dedifferentiation. Inhibition of TGF-β signaling expands β cell lineage in the development. However, deletion of Tgfbr1 has no influence on insulin demand-induced but abolishes inflammation-induced β cell proliferation. Among canonical TGF-β signaling, Smad3 but not Smad2 is the predominant repressor of β cell proliferation in response to systemic insulin demand. Deletion of Smad3 simultaneously improves β cell function, apoptosis, and systemic insulin resistance with the consequence of eliminated overt diabetes in diabetic mouse models, revealing Smad3 as a key mediator and ideal therapeutic target for type-2 diabetes. However, Smad7 shows controversial effects on β cell proliferation and glucose homeostasis in animal studies. On the other hand, overexpression of Tgfb1 prevents β cells from autoimmune destruction without influence on β cell function. All these findings reveal the diverse regulatory roles of TGF-β signaling in β cell biology.
Keywords: TGF-β signaling; apoptosis; dedifferentiation; diabetes; function; proliferation; β cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SMAD7 enhances adult β-cell proliferation without significantly affecting β-cell function in mice.J Biol Chem. 2020 Apr 10;295(15):4858-4869. doi: 10.1074/jbc.RA119.011011. Epub 2020 Mar 2. J Biol Chem. 2020. PMID: 32122971 Free PMC article.
-
Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function.J Biol Chem. 2009 May 1;284(18):12246-57. doi: 10.1074/jbc.M805379200. Epub 2009 Mar 5. J Biol Chem. 2009. PMID: 19265200 Free PMC article.
-
Smad3 deficiency promotes beta cell proliferation and function in db/db mice via restoring Pax6 expression.Theranostics. 2021 Jan 1;11(6):2845-2859. doi: 10.7150/thno.51857. eCollection 2021. Theranostics. 2021. PMID: 33456576 Free PMC article.
-
Diverse roles of TGF-β/Smads in renal fibrosis and inflammation.Int J Biol Sci. 2011;7(7):1056-67. doi: 10.7150/ijbs.7.1056. Epub 2011 Sep 2. Int J Biol Sci. 2011. PMID: 21927575 Free PMC article. Review.
-
TGF-β Signaling in Pancreatic Islet β Cell Development and Function.Endocrinology. 2021 Mar 1;162(3):bqaa233. doi: 10.1210/endocr/bqaa233. Endocrinology. 2021. PMID: 33349851 Free PMC article. Review.
Cited by
-
Unravelling the Crosstalk between Estrogen Deficiency and Gut-biotaDysbiosis in the Development of Diabetes Mellitus.Curr Diabetes Rev. 2024;20(10):e240124226067. doi: 10.2174/0115733998275953231129094057. Curr Diabetes Rev. 2024. PMID: 38275037 Review.
-
Efficacy and Mechanism of Alprostadil in Diabetes Mellitus Combined with Peripheral Atherosclerosis.Anatol J Cardiol. 2024 Sep 18;28(11):533-41. doi: 10.14744/AnatolJCardiol.2024.4582. Online ahead of print. Anatol J Cardiol. 2024. PMID: 39292156 Free PMC article.
-
Inflammation accelerating intestinal fibrosis: from mechanism to clinic.Eur J Med Res. 2024 Jun 18;29(1):335. doi: 10.1186/s40001-024-01932-2. Eur J Med Res. 2024. PMID: 38890719 Free PMC article. Review.
-
Activated Growth Factor From Platelets as Treatment for Diabetic Retinopathy Through Antioxidant-Oxidative Stress Pathway.Diabetes Metab Syndr Obes. 2025 Jan 31;18:305-313. doi: 10.2147/DMSO.S490055. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 39906695 Free PMC article.
-
Exploring TGF-β Signaling in Cancer Progression: Prospects and Therapeutic Strategies.Onco Targets Ther. 2025 Feb 18;18:233-262. doi: 10.2147/OTT.S493643. eCollection 2025. Onco Targets Ther. 2025. PMID: 39989503 Free PMC article. Review.
References
-
- WHO Diabetes Factsheet N. 312. [(accessed on 10 December 2021)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diabetes.
-
- Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

