Alcohol and Prostate Cancer: Time to Draw Conclusions
- PMID: 35327568
- PMCID: PMC8945566
- DOI: 10.3390/biom12030375
Alcohol and Prostate Cancer: Time to Draw Conclusions
Abstract
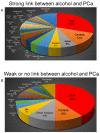
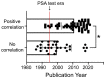
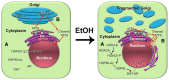
It has been a long-standing debate in the research and medical societies whether alcohol consumption is linked to the risk of prostate cancer (PCa). Many comprehensive studies from different geographical areas and nationalities have shown that moderate and heavy drinking is positively correlated with the development of PCa. Nevertheless, some observations could not confirm that such a correlation exists; some even suggest that wine consumption could prevent or slow prostate tumor growth. Here, we have rigorously analyzed the evidence both for and against the role of alcohol in PCa development. We found that many of the epidemiological studies did not consider other, potentially critical, factors, including diet (especially, low intake of fish, vegetables and linoleic acid, and excessive use of red meat), smoking, family history of PCa, low physical activity, history of high sexual activities especially with early age of first intercourse, and sexually transmitted infections. In addition, discrepancies between observations come from selectivity criteria for control groups, questionnaires about the type and dosage of alcohol, and misreported alcohol consumption. The lifetime history of alcohol consumption is critical given that a prostate tumor is typically slow-growing; however, many epidemiological observations that show no association monitored only current or relatively recent drinking status. Nevertheless, the overall conclusion is that high alcohol intake, especially binge drinking, is associated with increased risk for PCa, and this effect is not limited to any type of beverage. Alcohol consumption is also directly linked to PCa lethality as it may accelerate the growth of prostate tumors and significantly shorten the time for the progression to metastatic PCa. Thus, we recommend immediately quitting alcohol for patients diagnosed with PCa. We discuss the features of alcohol metabolism in the prostate tissue and the damaging effect of ethanol metabolites on intracellular organization and trafficking. In addition, we review the impact of alcohol consumption on prostate-specific antigen level and the risk for benign prostatic hyperplasia. Lastly, we highlight the known mechanisms of alcohol interference in prostate carcinogenesis and the possible side effects of alcohol during androgen deprivation therapy.
Keywords: alcohol consumption; ethanol metabolism; prostate cancer; prostate cancer-associated mortality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada.Cancer Epidemiol. 2016 Dec;45:11-17. doi: 10.1016/j.canep.2016.09.004. Epub 2016 Sep 22. Cancer Epidemiol. 2016. PMID: 27664387
-
Alcohol intake increases high-grade prostate cancer risk among men taking dutasteride in the REDUCE trial.Eur Urol. 2014 Dec;66(6):1133-8. doi: 10.1016/j.eururo.2014.01.037. Epub 2014 Feb 9. Eur Urol. 2014. PMID: 24568894 Free PMC article. Clinical Trial.
-
Alcohol and the risk of prostate cancer and benign prostatic hyperplasia.Urology. 2004 Oct;64(4):717-22. doi: 10.1016/j.urology.2004.05.002. Urology. 2004. PMID: 15491708
-
Latest Evidence on the Impact of Smoking, Sports, and Sexual Activity as Modifiable Lifestyle Risk Factors for Prostate Cancer Incidence, Recurrence, and Progression: A Systematic Review of the Literature by the European Association of Urology Section of Oncological Urology (ESOU).Eur Urol Focus. 2019 Sep;5(5):756-787. doi: 10.1016/j.euf.2018.02.007. Epub 2018 Mar 23. Eur Urol Focus. 2019. PMID: 29576530
-
Alcohol consumption and prostate cancer: a mini review.Exp Oncol. 2010 Jul;32(2):66-70. Exp Oncol. 2010. PMID: 20693964 Review.
Cited by
-
Causal Effects of Modifiable Behaviors on Prostate Cancer in Europeans and East Asians: A Comprehensive Mendelian Randomization Study.Biology (Basel). 2023 Apr 29;12(5):673. doi: 10.3390/biology12050673. Biology (Basel). 2023. PMID: 37237487 Free PMC article.
-
The Risk Factors and Screening Uptake for Prostate Cancer: A Scoping Review.Healthcare (Basel). 2023 Oct 20;11(20):2780. doi: 10.3390/healthcare11202780. Healthcare (Basel). 2023. PMID: 37893854 Free PMC article.
-
Lifestyle habits to prevent the development of benign prostatic hyperplasia: Analysis of Japanese nationwide datasets.Prostate Int. 2022 Dec;10(4):200-206. doi: 10.1016/j.prnil.2022.06.004. Epub 2022 Jun 28. Prostate Int. 2022. PMID: 36570647 Free PMC article.
-
Association between Alcohol Intake and Prostate Cancer Mortality and Survival.Nutrients. 2023 Feb 12;15(4):925. doi: 10.3390/nu15040925. Nutrients. 2023. PMID: 36839283 Free PMC article.
-
A Genome-Wide Association Study of Prostate Cancer Susceptibility Using Occupational and Environmental Factors as Confounding Factors.Cureus. 2024 Jan 25;16(1):e52926. doi: 10.7759/cureus.52926. eCollection 2024 Jan. Cureus. 2024. PMID: 38406143 Free PMC article.
References
-
- Teissedre P.-L., Rasines-Perea Z., Ruf J.-C., Stockley C., Antoce A.O., Romano R., Fradera U., Kosti R.I. Effects of alcohol consumption in general, and wine in particular, on the risk of cancer development: A review. OENO One. 2020;54:813–832. doi: 10.20870/oeno-one.2020.54.4.3569. - DOI
-
- Agarwal S.K. Alcohol and noncommunicable diseases: Part ii cancer, diabetes mellitus, kidney diseases, Alzheimer’s disease, arthritis. CMI. 2021;2:185–200.
-
- World Health Organization . Global Status Report on Alcohol and Health 2018. World Health Organization; Geneva, Switzerland: 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

