Mitochondrial Oxidative Stress and Cell Death in Podocytopathies
- PMID: 35327595
- PMCID: PMC8945726
- DOI: 10.3390/biom12030403
Mitochondrial Oxidative Stress and Cell Death in Podocytopathies
Abstract
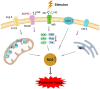
Podocytopathies are kidney diseases that are driven by podocyte injury with proteinuria and proteinuria-related symptoms as the main clinical presentations. Albeit podocytopathies are the major contributors to end-stage kidney disease, the underlying molecular mechanisms of podocyte injury remain to be elucidated. Mitochondrial oxidative stress is associated with kidney diseases, and increasing evidence suggests that oxidative stress plays a vital role in the pathogenesis of podocytopathies. Accumulating evidence has placed mitochondrial oxidative stress in the focus of cell death research. Excessive generated reactive oxygen species over antioxidant defense under pathological conditions lead to oxidative damage to cellular components and regulate cell death in the podocyte. Conversely, exogenous antioxidants can protect podocyte from cell death. This review provides an overview of the role of mitochondrial oxidative stress in podocytopathies and discusses its role in the cell death of the podocyte, aiming to identify the novel targets to improve the treatment of patients with podocytopathies.
Keywords: antioxidant defense; cell death; mitochondrial oxidative stress; podocytopathies; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeting oxidative stress-induced lipid peroxidation enhances podocyte function in cystinosis.J Transl Med. 2025 Feb 20;23(1):206. doi: 10.1186/s12967-024-05996-w. J Transl Med. 2025. PMID: 39980044 Free PMC article.
-
Biofactors regulating mitochondrial function and dynamics in podocytes and podocytopathies.J Cell Physiol. 2023 Oct;238(10):2206-2227. doi: 10.1002/jcp.31110. Epub 2023 Sep 2. J Cell Physiol. 2023. PMID: 37659096 Review.
-
Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria.Kidney Int. 2019 Apr;95(4):830-845. doi: 10.1016/j.kint.2018.10.032. Epub 2019 Feb 12. Kidney Int. 2019. PMID: 30770219 Free PMC article.
-
Nestin protects podocyte from injury in lupus nephritis by mitophagy and oxidative stress.Cell Death Dis. 2020 May 5;11(5):319. doi: 10.1038/s41419-020-2547-4. Cell Death Dis. 2020. PMID: 32371936 Free PMC article.
-
Targeting podocyte-associated diseases.Adv Drug Deliv Rev. 2010 Nov 30;62(14):1325-36. doi: 10.1016/j.addr.2010.08.012. Epub 2010 Sep 7. Adv Drug Deliv Rev. 2010. PMID: 20828590 Review.
Cited by
-
Rebamipide Attenuates Lupus Nephritis by Enhancing Antioxidative Defense in Podocytes: Evidence from a Lupus-Prone Mouse Model.Int J Mol Sci. 2025 Jun 17;26(12):5809. doi: 10.3390/ijms26125809. Int J Mol Sci. 2025. PMID: 40565270 Free PMC article.
-
Redox Imbalance and Mitochondrial Abnormalities in Kidney Disease.Biomolecules. 2022 Mar 21;12(3):476. doi: 10.3390/biom12030476. Biomolecules. 2022. PMID: 35327668 Free PMC article.
-
Nanodrugs alleviate acute kidney injury: Manipulate RONS at kidney.Bioact Mater. 2022 Sep 29;22:141-167. doi: 10.1016/j.bioactmat.2022.09.021. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36203963 Free PMC article. Review.
-
HR repair pathway plays a crucial role in maintaining neural stem cell fate under irradiation stress.Life Sci Alliance. 2023 May 17;6(8):e202201802. doi: 10.26508/lsa.202201802. Print 2023 Aug. Life Sci Alliance. 2023. PMID: 37197982 Free PMC article.
-
Targeting programmed cell death pathways: emerging therapeutic strategies for diabetic kidney disease.Front Endocrinol (Lausanne). 2025 Jun 11;16:1513895. doi: 10.3389/fendo.2025.1513895. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40568564 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

