A Multisensory Network Drives Nuclear Mechanoadaptation
- PMID: 35327596
- PMCID: PMC8945967
- DOI: 10.3390/biom12030404
A Multisensory Network Drives Nuclear Mechanoadaptation
Abstract
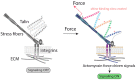
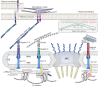
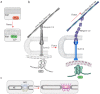
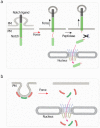
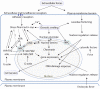
Cells have adapted to mechanical forces early in evolution and have developed multiple mechanisms ensuring sensing of, and adaptation to, the diversity of forces operating outside and within organisms. The nucleus must necessarily adapt to all types of mechanical signals, as its functions are essential for virtually all cell processes, many of which are tuned by mechanical cues. To sense forces, the nucleus is physically connected with the cytoskeleton, which senses and transmits forces generated outside and inside the cell. The nuclear LINC complex bridges the cytoskeleton and the nuclear lamina to transmit mechanical information up to the chromatin. This system creates a force-sensing macromolecular complex that, however, is not sufficient to regulate all nuclear mechanoadaptation processes. Within the nucleus, additional mechanosensitive structures, including the nuclear envelope and the nuclear pore complex, function to regulate nuclear mechanoadaptation. Similarly, extra nuclear mechanosensitive systems based on plasma membrane dynamics, mechanotransduce information to the nucleus. Thus, the nucleus has the intrinsic structural components needed to receive and interpret mechanical inputs, but also rely on extra nuclear mechano-sensors that activate nuclear regulators in response to force. Thus, a network of mechanosensitive cell structures ensures that the nucleus has a tunable response to mechanical cues.
Keywords: lipid bilayer mechano-sensing; mechano-transduction; mechanosensitive molecules; nuclear envelope; nucleus.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

