Mechanistic Investigation of GHS-R Mediated Glucose-Stimulated Insulin Secretion in Pancreatic Islets
- PMID: 35327599
- PMCID: PMC8945998
- DOI: 10.3390/biom12030407
Mechanistic Investigation of GHS-R Mediated Glucose-Stimulated Insulin Secretion in Pancreatic Islets
Abstract
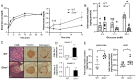
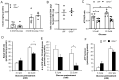
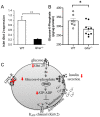
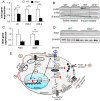
Ghrelin receptor, a growth hormone secretagogue receptor (GHS-R), is expressed in the pancreas. Emerging evidence indicates that GHS-R is involved in the regulation of glucose-stimulated insulin secretion (GSIS), but the mechanism by which GHS-R regulates GSIS in the pancreas is unclear. In this study, we investigated the role of GHS-R on GSIS in detail using global Ghsr-/- mice (in vivo) and Ghsr-ablated pancreatic islets (ex vivo). GSIS was attenuated in both Ghsr-/- mice and Ghsr-ablated islets, while the islet morphology was similar between WT and Ghsr-/- mice. To elucidate the mechanism underpinning Ghsr-mediated GSIS, we investigated the key steps of the GSIS signaling cascade. The gene expression of glucose transporter 2 (Glut2) and the glucose-metabolic intermediate-glucose-6-phosphate (G6P) were reduced in Ghsr-ablated islets, supporting decreased glucose uptake. There was no difference in mitochondrial DNA content in the islets of WT and Ghsr-/- mice, but the ATP/ADP ratio in Ghsr-/- islets was significantly lower than that of WT islets. Moreover, the expression of pancreatic and duodenal homeobox 1 (Pdx1), as well as insulin signaling genes of insulin receptor (IR) and insulin receptor substrates 1 and 2 (IRS1/IRS2), was downregulated in Ghsr-/- islets. Akt is the key mediator of the insulin signaling cascade. Concurrently, Akt phosphorylation was reduced in the pancreas of Ghsr-/- mice under both insulin-stimulated and homeostatic conditions. These findings demonstrate that GHS-R ablation affects key components of the insulin signaling pathway in the pancreas, suggesting the existence of a cross-talk between GHS-R and the insulin signaling pathway in pancreatic islets, and GHS-R likely regulates GSIS via the Akt-Pdx1-GLUT2 pathway.
Keywords: glucose transporter 2 (Glut2); glucose-stimulated insulin secretion (GSIS); growth hormone secretagogue receptor (GHS-R); insulin; islets; pancreatic and duodenal homeobox 1 (Pdx1).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Obestatin stimulates glucose-induced insulin secretion through ghrelin receptor GHS-R.Sci Rep. 2017 Apr 20;7(1):979. doi: 10.1038/s41598-017-00888-0. Sci Rep. 2017. PMID: 28428639 Free PMC article.
-
β Cell GHS-R Regulates Insulin Secretion and Sensitivity.Int J Mol Sci. 2021 Apr 11;22(8):3950. doi: 10.3390/ijms22083950. Int J Mol Sci. 2021. PMID: 33920473 Free PMC article.
-
Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice.Am J Physiol Endocrinol Metab. 2012 Aug 1;303(3):E422-31. doi: 10.1152/ajpendo.00576.2011. Epub 2012 Jun 5. Am J Physiol Endocrinol Metab. 2012. PMID: 22669248 Free PMC article.
-
Ghrelin signalling in β-cells regulates insulin secretion and blood glucose.Diabetes Obes Metab. 2014 Sep;16 Suppl 1:111-7. doi: 10.1111/dom.12344. Diabetes Obes Metab. 2014. PMID: 25200304 Review.
-
Status of ghrelin as an islet hormone and paracrine/autocrine regulator of insulin secretion.Peptides. 2022 Feb;148:170681. doi: 10.1016/j.peptides.2021.170681. Epub 2021 Oct 30. Peptides. 2022. PMID: 34728253 Review.
Cited by
-
Germline and conditional ghrelin knockout increases islet size.J Clin Invest. 2023 Dec 15;133(24):e175799. doi: 10.1172/JCI175799. J Clin Invest. 2023. PMID: 38099493 Free PMC article.
-
Machine learning chained neural network analysis of oxygen transport amplifies the physiological relevance of vascularized microphysiological systems.Bioeng Transl Med. 2023 Aug 1;8(6):e10582. doi: 10.1002/btm2.10582. eCollection 2023 Nov. Bioeng Transl Med. 2023. PMID: 38023704 Free PMC article.
-
2-Hydroxylation is a chemical switch linking fatty acids to glucose-stimulated insulin secretion.J Biol Chem. 2024 Dec;300(12):107912. doi: 10.1016/j.jbc.2024.107912. Epub 2024 Oct 21. J Biol Chem. 2024. PMID: 39442620 Free PMC article.
-
The expression and function of growth hormone secretagogue receptor in immune cells: A current perspective.Exp Biol Med (Maywood). 2022 Dec;247(24):2184-2191. doi: 10.1177/15353702221121635. Epub 2022 Sep 23. Exp Biol Med (Maywood). 2022. PMID: 36151745 Free PMC article. Review.
-
GHSR Deletion in β-Cells of Male Mice: Ineffective in Obesity, but Effective in Protecting against Streptozotocin-Induced β-Cell Injury in Aging.Nutrients. 2024 May 13;16(10):1464. doi: 10.3390/nu16101464. Nutrients. 2024. PMID: 38794702 Free PMC article.
References
-
- Date Y., Kojima M., Hosoda H., Sawaguchi A., Mondal M.S., Suganuma T., Matsukura S., Kangawa K., Nakazato M. Ghrelin, a Novel Growth Hormone-Releasing Acylated Peptide, Is Synthesized in a Distinct Endocrine Cell Type in the Gastrointestinal Tracts of Rats and Humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. - DOI - PubMed
-
- Granata R., Settanni F., Gallo D., Trovato L., Biancone L., Cantaluppi V., Nano R., Annunziata M., Campiglia P., Arnoletti E., et al. Obestatin Promotes Survival of Pancreatic Beta-Cells and Human Islets and Induces Expression of Genes Involved in the Regulation of -Cell Mass and Function. Diabetes. 2007;57:967–979. doi: 10.2337/db07-1104. - DOI - PubMed
-
- Volante M., Allìa E., Gugliotta P., Funaro A., Broglio F., Deghenghi R., Muccioli G., Ghigo E., Papotti M. Expression of Ghrelin and of the GH Secretagogue Receptor by Pancreatic Islet Cells and Related Endocrine Tumors. J. Clin. Endocrinol. Metab. 2002;87:1300–1308. doi: 10.1210/jcem.87.3.8279. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

