DNA Methylation Malleability and Dysregulation in Cancer Progression: Understanding the Role of PARP1
- PMID: 35327610
- PMCID: PMC8946700
- DOI: 10.3390/biom12030417
DNA Methylation Malleability and Dysregulation in Cancer Progression: Understanding the Role of PARP1
Abstract
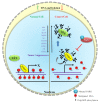
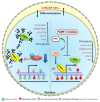
Mammalian genomic DNA methylation represents a key epigenetic modification and its dynamic regulation that fine-tunes the gene expression of multiple pathways during development. It maintains the gene expression of one generation of cells; particularly, the mitotic inheritance of gene-expression patterns makes it the key governing mechanism of epigenetic change to the next generation of cells. Convincing evidence from recent discoveries suggests that the dynamic regulation of DNA methylation is accomplished by the enzymatic action of TET dioxygenase, which oxidizes the methyl group of cytosine and activates transcription. As a result of aberrant DNA modifications, genes are improperly activated or inhibited in the inappropriate cellular context, contributing to a plethora of inheritable diseases, including cancer. We outline recent advancements in understanding how DNA modifications contribute to tumor suppressor gene silencing or oncogenic-gene stimulation, as well as dysregulation of DNA methylation in cancer progression. In addition, we emphasize the function of PARP1 enzymatic activity or inhibition in the maintenance of DNA methylation dysregulation. In the context of cancer remediation, the impact of DNA methylation and PARP1 pharmacological inhibitors, and their relevance as a combination therapy are highlighted.
Keywords: DNA demethylases; DNA demethylases inhibitors; DNA methylation; PARP1; cancer cells; oncogene; poly(ADP-ribose); tumor progression; tumor suppressor gene.
Conflict of interest statement
The authors declare no conflict of interest with respect to the authorship and publication of this article.
Figures
Similar articles
-
Parp1 localizes within the Dnmt1 promoter and protects its unmethylated state by its enzymatic activity.PLoS One. 2009;4(3):e4717. doi: 10.1371/journal.pone.0004717. Epub 2009 Mar 5. PLoS One. 2009. PMID: 19262751 Free PMC article.
-
ADP-ribose polymers localized on Ctcf-Parp1-Dnmt1 complex prevent methylation of Ctcf target sites.Biochem J. 2012 Jan 15;441(2):645-52. doi: 10.1042/BJ20111417. Biochem J. 2012. PMID: 21985173 Free PMC article.
-
Promoter hypomethylation, especially around the E26 transformation-specific motif, and increased expression of poly (ADP-ribose) polymerase 1 in BRCA-mutated serous ovarian cancer.BMC Cancer. 2013 Feb 26;13:90. doi: 10.1186/1471-2407-13-90. BMC Cancer. 2013. PMID: 23442605 Free PMC article.
-
Poly(ADP-Ribose) Polymerase 1: Cellular Pluripotency, Reprogramming, and Tumorogenesis.Int J Mol Sci. 2015 Jul 9;16(7):15531-45. doi: 10.3390/ijms160715531. Int J Mol Sci. 2015. PMID: 26184161 Free PMC article. Review.
-
[Molecular mechanisms of regulaion of transcription by PARP1].Mol Biol (Mosk). 2015 Jan-Feb;49(1):99-113. Mol Biol (Mosk). 2015. PMID: 25916114 Review. Russian.
Cited by
-
Structural basis of water-mediated cis Watson-Crick/Hoogsteen base-pair formation in non-CpG methylation.Nucleic Acids Res. 2024 Aug 12;52(14):8566-8579. doi: 10.1093/nar/gkae594. Nucleic Acids Res. 2024. PMID: 38989613 Free PMC article.
-
The protective effect of 17 β-estradiol on human uterosacral ligament fibroblasts from postmenopausal women with pelvic organ prolapse.Front Physiol. 2022 Oct 10;13:980843. doi: 10.3389/fphys.2022.980843. eCollection 2022. Front Physiol. 2022. PMID: 36299259 Free PMC article.
-
Comprehensive pan-cancer analysis reveals ENC1 as a promising prognostic biomarker for tumor microenvironment and therapeutic responses.Sci Rep. 2024 Oct 25;14(1):25331. doi: 10.1038/s41598-024-76798-9. Sci Rep. 2024. PMID: 39455818 Free PMC article.
-
Unveiling the microbial orchestra: exploring the role of microbiota in cancer development and treatment.Discov Oncol. 2025 Apr 30;16(1):646. doi: 10.1007/s12672-025-02352-2. Discov Oncol. 2025. PMID: 40304829 Free PMC article. Review.
-
Epigenetic modifications: Critical participants of the PD‑L1 regulatory mechanism in solid tumors (Review).Int J Oncol. 2022 Nov;61(5):134. doi: 10.3892/ijo.2022.5424. Epub 2022 Sep 21. Int J Oncol. 2022. PMID: 36129152 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

