Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia
- PMID: 35327614
- PMCID: PMC8946184
- DOI: 10.3390/biom12030422
Progesterone Attenuates SIRT1-Deficiency-Mediated Pre-Eclampsia
Abstract
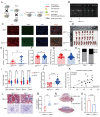
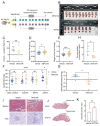
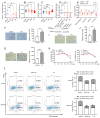
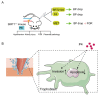
Pre-eclampsia is a severe hypertensive disorder of pregnancy (HDP), mainly characterized by new-onset hypertension with proteinuria after 20-week gestation. Sirtuin1 (SIRT1), a class III histone deacetylase, is associated with the regulation of various pathophysiological processes, including inflammation, immune response, metabolism, and autophagy. However, the effect of SIRT1 in the pathogenesis of pre-eclampsia remains to be elucidated. In this study, we found that the expression of SIRT1 was relatively lower in the placentas and serum samples of pre-eclampsia patients. Typical pre-eclampsia-like symptoms, such as hypertension, proteinuria, fetal growth restriction, kidney injury, and a narrow placental labyrinth layer, were observed in SIRT1 knockdown (SIRT1+/-) mice. Of note, these performances could be improved after the intraperitoneal injection of SIRT1 agonist SRT2104. More importantly, we found that the efficacy of progesterone on attenuating symptoms of PE was profoundly better than that of metformin in SIRT1+/- mice. In addition, our results suggested that progesterone can promote the invasion and inhibit the apoptosis of trophoblasts. These data suggest that SIRT1 plays an important role in pre-eclampsia and that progesterone alleviates pre-eclampsia-like symptoms mediated by SIRT1 deficiency.
Keywords: SIRT1; SRT2104; metformin; pre-eclampsia; pre-eclampsia-like mice; progesterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P., Saito S., Hall D.R., Warren C.E., Adoyi G., Ishaku S., et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. - DOI - PubMed
-
- Goel A., Maski M.R., Bajracharya S., Wenger J.B., Zhang D., Salahuddin S., Shahul S.S., Thadhani R., Seely E.W., Karumanchi S.A., et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation. 2015;132:1726–1733. doi: 10.1161/CIRCULATIONAHA.115.015721. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

