ENT-A010, a Novel Steroid Derivative, Displays Neuroprotective Functions and Modulates Microglial Responses
- PMID: 35327616
- PMCID: PMC8946810
- DOI: 10.3390/biom12030424
ENT-A010, a Novel Steroid Derivative, Displays Neuroprotective Functions and Modulates Microglial Responses
Abstract
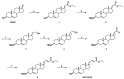
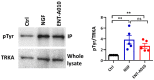
Tackling neurodegeneration and neuroinflammation is particularly challenging due to the complexity of central nervous system (CNS) disorders, as well as the limited drug accessibility to the brain. The activation of tropomyosin-related kinase A (TRKA) receptor signaling by the nerve growth factor (NGF) or the neurosteroid dehydroepiandrosterone (DHEA) may combat neurodegeneration and regulate microglial function. In the present study, we synthesized a C-17-spiro-cyclopropyl DHEA derivative (ENT-A010), which was capable of activating TRKA. ENT-A010 protected PC12 cells against serum starvation-induced cell death, dorsal root ganglia (DRG) neurons against NGF deprivation-induced apoptosis and hippocampal neurons against Aβ-induced apoptosis. In addition, ENT-A010 pretreatment partially restored homeostatic features of microglia in the hippocampus of lipopolysaccharide (LPS)-treated mice, enhanced Aβ phagocytosis, and increased Ngf expression in microglia in vitro. In conclusion, the small molecule ENT-A010 elicited neuroprotective effects and modulated microglial function, thereby emerging as an interesting compound, which merits further study in the treatment of CNS disorders.
Keywords: Aβ; DHEA; ENT-A010; hippocampus; microglia; neuroprotection; phagocytosis; ΤRKA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
DHEA Attenuates Microglial Activation via Induction of JMJD3 in Experimental Subarachnoid Haemorrhage.J Neuroinflammation. 2019 Nov 28;16(1):243. doi: 10.1186/s12974-019-1641-y. J Neuroinflammation. 2019. PMID: 31779639 Free PMC article.
-
Differential effects of dehydroepiandrosterone and testosterone in prostate and colon cancer cell apoptosis: the role of nerve growth factor (NGF) receptors.Endocrinology. 2013 Jul;154(7):2446-56. doi: 10.1210/en.2012-2249. Epub 2013 May 21. Endocrinology. 2013. PMID: 23696568
-
Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482
-
Painless Nerve Growth Factor: A TrkA biased agonist mediating a broad neuroprotection via its actions on microglia cells.Pharmacol Res. 2019 Jan;139:17-25. doi: 10.1016/j.phrs.2018.10.028. Epub 2018 Nov 1. Pharmacol Res. 2019. PMID: 30391352 Review.
-
A Microglial Function for the Nerve Growth Factor: Predictions of the Unpredictable.Cells. 2022 Jun 3;11(11):1835. doi: 10.3390/cells11111835. Cells. 2022. PMID: 35681529 Free PMC article. Review.
Cited by
-
Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury.Biomedicines. 2023 Apr 13;11(4):1170. doi: 10.3390/biomedicines11041170. Biomedicines. 2023. PMID: 37189788 Free PMC article.
-
A quest for the stereo-electronic requirements for selective agonism for the neurotrophin receptors TrkA and TrkB in 17-spirocyclic-dehydroepiandrosterone derivatives.Front Mol Neurosci. 2023 Sep 28;16:1244133. doi: 10.3389/fnmol.2023.1244133. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37840771 Free PMC article.
-
Anti-apoptotic effects of the medicinal plant Sterculia setigera in a model of serum deprivation-induced PC12 cells death.IBRO Neurosci Rep. 2025 Jun 16;19:110-116. doi: 10.1016/j.ibneur.2025.06.007. eCollection 2025 Dec. IBRO Neurosci Rep. 2025. PMID: 40600168 Free PMC article.
-
Small-Molecule Trk Agonists: Where Do We Go from Here?J Med Chem. 2025 Aug 14;68(15):15233-15259. doi: 10.1021/acs.jmedchem.4c02365. Epub 2025 Jul 18. J Med Chem. 2025. PMID: 40679347 Free PMC article. Review.
-
Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health.Int J Mol Sci. 2024 Nov 12;25(22):12155. doi: 10.3390/ijms252212155. Int J Mol Sci. 2024. PMID: 39596221 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

