Ketamine Improves Desensitization of µ-Opioid Receptors Induced by Repeated Treatment with Fentanyl but Not with Morphine
- PMID: 35327617
- PMCID: PMC8946650
- DOI: 10.3390/biom12030426
Ketamine Improves Desensitization of µ-Opioid Receptors Induced by Repeated Treatment with Fentanyl but Not with Morphine
Abstract
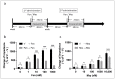
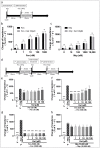
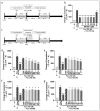
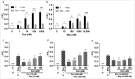
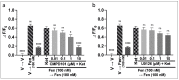
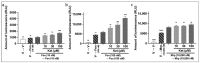
The issue of tolerance to continuous or repeated administration of opioids should be addressed. The ability of ketamine to improve opioid tolerance has been reported in clinical studies, and its mechanism of tolerance may involve improved desensitization of μ-opioid receptors (MORs). We measured changes in MOR activity and intracellular signaling induced by repeated fentanyl and morphine administration and investigated the effects of ketamine on these changes with human embryonic kidney 293 cells expressing MOR using the CellKey™, cADDis cyclic adenosine monophosphate, and PathHunter® β-arrestin recruitment assays. Repeated administration of fentanyl or morphine suppressed the second MOR responses. Administration of ketamine before a second application of opioids within clinical concentrations improved acute desensitization and enhanced β-arrestin recruitment elicited by fentanyl but not by morphine. The effects of ketamine on fentanyl were suppressed by co-treatment with an inhibitor of G-protein-coupled receptor kinase (GRK). Ketamine may potentially reduce fentanyl tolerance but not that of morphine through modulation of GRK-mediated pathways, possibly changing the conformational changes of β-arrestin to MOR.
Keywords: G protein receptor kinase; desensitization; fentanyl; ketamine; morphine; tolerance; µ-opioid receptor; β-arrestin.
Conflict of interest statement
Y.U. received financial support from Daiichi Sankyo Co., Ltd. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Distinct Profiles of Desensitization of µ-Opioid Receptors Caused by Remifentanil or Fentanyl: In Vitro Assay with Cells and Three-Dimensional Structural Analyses.Int J Mol Sci. 2023 May 6;24(9):8369. doi: 10.3390/ijms24098369. Int J Mol Sci. 2023. PMID: 37176075 Free PMC article.
-
Usefulness for the combination of G-protein- and β-arrestin-biased ligands of μ-opioid receptors: Prevention of antinociceptive tolerance.Mol Pain. 2017 Jan-Dec;13:1744806917740030. doi: 10.1177/1744806917740030. Mol Pain. 2017. PMID: 29056067 Free PMC article.
-
Novel Opioid Analgesics for the Development of Transdermal Opioid Patches That Possess Morphine-Like Pharmacological Profiles Rather Than Fentanyl: Possible Opioid Switching Alternatives Among Patch Formula.Anesth Analg. 2022 May 1;134(5):1082-1093. doi: 10.1213/ANE.0000000000005954. Anesth Analg. 2022. PMID: 35427270 Free PMC article.
-
The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance.Anesth Analg. 2005 Sep;101(3):728-734. doi: 10.1213/01.ANE.0000160588.32007.AD. Anesth Analg. 2005. PMID: 16115983 Review.
-
[Development of opioid tolerance -- molecular mechanisms and clinical consequences].Anasthesiol Intensivmed Notfallmed Schmerzther. 2003 Jan;38(1):14-26. doi: 10.1055/s-2003-36558. Anasthesiol Intensivmed Notfallmed Schmerzther. 2003. PMID: 12522725 Review. German.
Cited by
-
Recent advances in the study of anesthesia-and analgesia-related mechanisms of S-ketamine.Front Pharmacol. 2023 Sep 14;14:1228895. doi: 10.3389/fphar.2023.1228895. eCollection 2023. Front Pharmacol. 2023. PMID: 37781698 Free PMC article. Review.
-
Distinct Profiles of Desensitization of µ-Opioid Receptors Caused by Remifentanil or Fentanyl: In Vitro Assay with Cells and Three-Dimensional Structural Analyses.Int J Mol Sci. 2023 May 6;24(9):8369. doi: 10.3390/ijms24098369. Int J Mol Sci. 2023. PMID: 37176075 Free PMC article.
-
Targeting Excitatory Glutamate Receptors for Morphine Tolerance: A Narrative Review.CNS Neurosci Ther. 2025 Jun;31(6):e70468. doi: 10.1111/cns.70468. CNS Neurosci Ther. 2025. PMID: 40484988 Free PMC article. Review.
References
-
- World Health Organization . Cancer Pain Relief: With a Guide to Opioid Availability. World Health Organization; Geneva, Switzerland: 1996.
-
- Chou R., Gordon D.B., de Leon-Casasola O.A., Rosenberg J.M., Bickler S., Brennan T., Carter T., Cassidy C.L., Chittenden E.H., Degenhardt E., et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. - DOI - PubMed
-
- Devlin J.W., Skrobik Y., Gélinas C., Needham D.M., Slooter A.J.C., Pandharipande P.P., Watson P.L., Weinhouse G.L., Nunnally M.E., Rochwerg B., et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

