Functional and Conformational Plasticity of an Animal Group 1 LEA Protein
- PMID: 35327618
- PMCID: PMC8946055
- DOI: 10.3390/biom12030425
Functional and Conformational Plasticity of an Animal Group 1 LEA Protein
Abstract
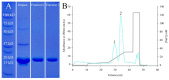
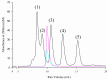
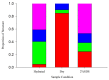
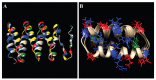
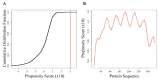
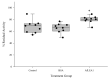
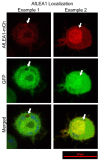
Group 1 (Dur-19, PF00477, LEA_5) Late Embryogenesis Abundant (LEA) proteins are present in organisms from all three domains of life, Archaea, Bacteria, and Eukarya. Surprisingly, Artemia is the only genus known to include animals that express group 1 LEA proteins in their desiccation-tolerant life-history stages. Bioinformatics analysis of circular dichroism data indicates that the group 1 LEA protein AfLEA1 is surprisingly ordered in the hydrated state and undergoes during desiccation one of the most pronounced disorder-to-order transitions described for LEA proteins from A. franciscana. The secondary structure in the hydrated state is dominated by random coils (42%) and β-sheets (35%) but converts to predominately α-helices (85%) when desiccated. Interestingly, AfLEA1 interacts with other proteins and nucleic acids, and RNA promotes liquid-liquid phase separation (LLPS) of the protein from the solvent during dehydration in vitro. Furthermore, AfLEA1 protects the enzyme lactate dehydrogenase (LDH) during desiccation but does not aid in restoring LDH activity after desiccation-induced inactivation. Ectopically expressed in D. melanogaster Kc167 cells, AfLEA1 localizes predominantly to the cytosol and increases the cytosolic viscosity during desiccation compared to untransfected control cells. Furthermore, the protein formed small biomolecular condensates in the cytoplasm of about 38% of Kc167 cells. These findings provide additional evidence for the hypothesis that the formation of biomolecular condensates to promote water stress tolerance during anhydrobiosis may be a shared feature across several groups of LEA proteins that display LLPS behaviors.
Keywords: LLPS; cryptobiosis; extremophiles; late embryogenesis abundant; protein condensate; water stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

