The Mechanisms of Restenosis and Relevance to Next Generation Stent Design
- PMID: 35327622
- PMCID: PMC8945897
- DOI: 10.3390/biom12030430
The Mechanisms of Restenosis and Relevance to Next Generation Stent Design
Abstract
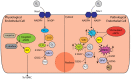
Stents are lifesaving mechanical devices that re-establish essential blood flow to the coronary circulation after significant vessel occlusion due to coronary vessel disease or thrombolytic blockade. Improvements in stent surface engineering over the last 20 years have seen significant reductions in complications arising due to restenosis and thrombosis. However, under certain conditions such as diabetes mellitus (DM), the incidence of stent-mediated complications remains 2-4-fold higher than seen in non-diabetic patients. The stents with the largest market share are designed to target the mechanisms behind neointimal hyperplasia (NIH) through anti-proliferative drugs that prevent the formation of a neointima by halting the cell cycle of vascular smooth muscle cells (VSMCs). Thrombosis is treated through dual anti-platelet therapy (DAPT), which is the continual use of aspirin and a P2Y12 inhibitor for 6-12 months. While the most common stents currently in use are reasonably effective at treating these complications, there is still significant room for improvement. Recently, inflammation and redox stress have been identified as major contributing factors that increase the risk of stent-related complications following percutaneous coronary intervention (PCI). The aim of this review is to examine the mechanisms behind inflammation and redox stress through the lens of PCI and its complications and to establish whether tailored targeting of these key mechanistic pathways offers improved outcomes for patients, particularly those where stent placement remains vulnerable to complications. In summary, our review highlights the most recent and promising research being undertaken in understanding the mechanisms of redox biology and inflammation in the context of stent design. We emphasize the benefits of a targeted mechanistic approach to decrease all-cause mortality, even in patients with diabetes.
Keywords: drug-eluting stents; inflammation; neointimal hyperplasia; redox; restenosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . Cardiovascular Diseases (CVDs) World Health Organisation; Geneva, Switzerland: 2017.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

