Continuous Low-Intensity Ultrasound Preserves Chondrogenesis of Mesenchymal Stromal Cells in the Presence of Cytokines by Inhibiting NFκB Activation
- PMID: 35327626
- PMCID: PMC8946190
- DOI: 10.3390/biom12030434
Continuous Low-Intensity Ultrasound Preserves Chondrogenesis of Mesenchymal Stromal Cells in the Presence of Cytokines by Inhibiting NFκB Activation
Abstract
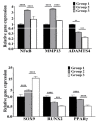
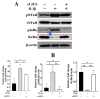
Proinflammatory joint environment, coupled with impeded chondrogenic differentiation of mesenchymal stromal cells (MSCs), led to inferior cartilage repair outcomes. Nuclear translocation of phosphorylated-NFκB downregulates SOX9 and hinders the chondrogenesis of MSCs. Strategies that minimize the deleterious effects of NFκB, while promoting MSC chondrogenesis, are of interest. This study establishes the ability of continuous low-intensity ultrasound (cLIUS) to preserve MSC chondrogenesis in a proinflammatory environment. MSCs were seeded in alginate:collagen hydrogels and cultured for 21 days in an ultrasound-assisted bioreactor (5.0 MHz, 2.5 Vpp; 4 applications/day) in the presence of IL1β and evaluated by qRT-PCR and immunofluorescence. The differential expression of markers associated with the NFκB pathway was assessed upon a single exposure of cLIUS and assayed by Western blotting, qRT-PCR, and immunofluorescence. Mitochondrial potential was evaluated by tetramethylrhodamine methyl ester (TMRM) assay. The chondroinductive potential of cLIUS was noted by the increased expression of SOX9 and COLII. cLIUS extended its chondroprotective effects by stabilizing the NFκB complex in the cytoplasm via engaging the IκBα feedback mechanism, thus preventing its nuclear translocation. cLIUS acted as a mitochondrial protective agent by restoring the mitochondrial potential and the mitochondrial mRNA expression in a proinflammatory environment. Altogether, our results demonstrated the potential of cLIUS for cartilage repair and regeneration under proinflammatory conditions.
Keywords: NFκB pathway; mesenchymal stromal cells; mitochondrial potential; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Filardo G., Vannini F., Marcacci M., Andriolo L., Ferruzzi A., Giannini S., Kon E. Matrix-assisted autologous chondrocyte transplantation for cartilage regeneration in osteoarthritic knees: Results and failures at midterm follow-up. Am. J. Sports Med. 2012;41:95–100. doi: 10.1177/0363546512463675. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

