Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice
- PMID: 35327639
- PMCID: PMC8946515
- DOI: 10.3390/biom12030447
Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice
Abstract
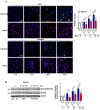
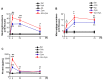
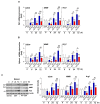
Signal transducer and activator of transcription 6 (STAT6) promotes an anti-inflammatory process by inducing the development of M2 macrophages. We investigated whether modulating STAT6 activity in macrophages using AS1517499, the specific STAT6 inhibitor, affects the restoration of homeostasis after an inflammatory insult by regulating PPARγ expression and activity. Administration of AS1517499 suppressed the enhanced STAT6 phosphorylation and nuclear translocation observed in peritoneal macrophages after zymosan injection. In addition, AS1517499 delayed resolution of acute inflammation as evidenced by enhanced secretion of pro-inflammatory cytokines, reduced secretion of anti-inflammatory cytokines in PLF and supernatants from peritoneal macrophages, and exaggerated neutrophil numbers and total protein levels in PLF. We demonstrate temporal increases in annexin A1 (AnxA1) protein and mRNA levels in peritoneal lavage fluid (PLF), peritoneal macrophages, and spleen in a murine model of zymosan-induced acute peritonitis. In vitro priming of mouse bone marrow-derived macrophages (BMDM) and peritoneal macrophages with AnxA1 induced STAT6 activation with enhanced PPARγ expression and activity. Using AS1517499, we demonstrate that inhibition of STAT6 activation delayed recovery of PPARγ expression and activity, as well as impaired efferocytosis. Taken together, these results suggest that activation of the STAT6 signaling pathway mediates PPARγ expression and activation in macrophages to resolve acute inflammation.
Keywords: AS1517499; PPARγ; acute peritonitis; annexin A1; macrophages.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
STAT6 Signaling Mediates PPARγ Activation and Resolution of Acute Sterile Inflammation in Mice.Cells. 2021 Feb 26;10(3):501. doi: 10.3390/cells10030501. Cells. 2021. PMID: 33652833 Free PMC article.
-
A STAT6 Inhibitor AS1517499 Reduces Preventive Effects of Apoptotic Cell Instillation on Bleomycin-Induced Lung Fibrosis by Suppressing PPARγ.Cell Physiol Biochem. 2018;45(5):1863-1877. doi: 10.1159/000487877. Epub 2018 Feb 28. Cell Physiol Biochem. 2018. PMID: 29510393
-
Pro-inflammatory cytokines negatively regulate PPARγ mediated gene expression in both human and murine macrophages via multiple mechanisms.Immunobiology. 2013 Nov;218(11):1336-44. doi: 10.1016/j.imbio.2013.06.011. Epub 2013 Jul 1. Immunobiology. 2013. PMID: 23870825
-
Pharmacological Inhibition of STAT6 Ameliorates Myeloid Fibroblast Activation and Alternative Macrophage Polarization in Renal Fibrosis.Front Immunol. 2021 Aug 26;12:735014. doi: 10.3389/fimmu.2021.735014. eCollection 2021. Front Immunol. 2021. PMID: 34512669 Free PMC article.
-
Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARγ and Nrf2 signaling.Free Radic Biol Med. 2023 May 20;201:98-110. doi: 10.1016/j.freeradbiomed.2023.03.014. Epub 2023 Mar 20. Free Radic Biol Med. 2023. PMID: 36940733
Cited by
-
Human Tendon-on-a-Chip for Modeling the Myofibroblast Microenvironment in Peritendinous Fibrosis.Adv Healthc Mater. 2025 Feb;14(4):e2403116. doi: 10.1002/adhm.202403116. Epub 2024 Nov 15. Adv Healthc Mater. 2025. PMID: 39544139 Free PMC article.
-
Zymosan-Induced Murine Peritonitis Is Associated with an Increased Sphingolipid Synthesis without Changing the Long to Very Long Chain Ceramide Ratio.Int J Mol Sci. 2023 Feb 1;24(3):2773. doi: 10.3390/ijms24032773. Int J Mol Sci. 2023. PMID: 36769096 Free PMC article.
-
Jak2/STAT6/c-Myc pathway is vital to the pathogenicity of Philadelphia-positive acute lymphoblastic leukemia caused by P190BCR-ABL.Cell Commun Signal. 2023 Jan 31;21(1):27. doi: 10.1186/s12964-023-01039-x. Cell Commun Signal. 2023. PMID: 36721266 Free PMC article.
-
The Role of STATs in Ovarian Cancer: Exploring Their Potential for Therapy.Cancers (Basel). 2023 Apr 26;15(9):2485. doi: 10.3390/cancers15092485. Cancers (Basel). 2023. PMID: 37173951 Free PMC article. Review.
-
Dendrobium officinale polysaccharide Converts M2 into M1 Subtype Macrophage Polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 Signaling Pathways to Inhibit Gastric Cancer.Molecules. 2023 Oct 12;28(20):7062. doi: 10.3390/molecules28207062. Molecules. 2023. PMID: 37894541 Free PMC article.
References
-
- Bhattacharjee A., Shukla M., Yakubenko V.P., Mulya A., Kundu S., Cathcart M.K. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic. Biol. Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

