Bifurcations and Proarrhythmic Behaviors in Cardiac Electrical Excitations
- PMID: 35327651
- PMCID: PMC8946197
- DOI: 10.3390/biom12030459
Bifurcations and Proarrhythmic Behaviors in Cardiac Electrical Excitations
Abstract
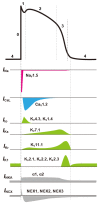
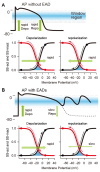
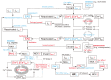
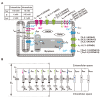
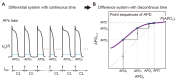
The heart is a hierarchical dynamic system consisting of molecules, cells, and tissues, and acts as a pump for blood circulation. The pumping function depends critically on the preceding electrical activity, and disturbances in the pattern of excitation propagation lead to cardiac arrhythmia and pump failure. Excitation phenomena in cardiomyocytes have been modeled as a nonlinear dynamical system. Because of the nonlinearity of excitation phenomena, the system dynamics could be complex, and various analyses have been performed to understand the complex dynamics. Understanding the mechanisms underlying proarrhythmic responses in the heart is crucial for developing new ways to prevent and control cardiac arrhythmias and resulting contractile dysfunction. When the heart changes to a pathological state over time, the action potential (AP) in cardiomyocytes may also change to a different state in shape and duration, often undergoing a qualitative change in behavior. Such a dynamic change is called bifurcation. In this review, we first summarize the contribution of ion channels and transporters to AP formation and our knowledge of ion-transport molecules, then briefly describe bifurcation theory for nonlinear dynamical systems, and finally detail its recent progress, focusing on the research that attempts to understand the developing mechanisms of abnormal excitations in cardiomyocytes from the perspective of bifurcation phenomena.
Keywords: action potential; afterdepolarizations; bifurcation theory; cardiac arrhythmias; nonlinear dynamical system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve.Cardiovasc Res. 2013 Jul 1;99(1):6-15. doi: 10.1093/cvr/cvt104. Epub 2013 Apr 25. Cardiovasc Res. 2013. PMID: 23619423 Free PMC article. Review.
-
Period doubling cascades of limit cycles in cardiac action potential models as precursors to chaotic early Afterdepolarizations.BMC Syst Biol. 2017 Apr 4;11(1):42. doi: 10.1186/s12918-017-0422-4. BMC Syst Biol. 2017. PMID: 28376924 Free PMC article.
-
Intracellular ion accumulation in the genesis of complex action potential dynamics under cardiac diseases.Phys Rev E. 2024 Feb;109(2-1):024410. doi: 10.1103/PhysRevE.109.024410. Phys Rev E. 2024. PMID: 38491656 Free PMC article.
-
Computational approaches to understand cardiac electrophysiology and arrhythmias.Am J Physiol Heart Circ Physiol. 2012 Oct 1;303(7):H766-83. doi: 10.1152/ajpheart.01081.2011. Epub 2012 Aug 10. Am J Physiol Heart Circ Physiol. 2012. PMID: 22886409 Free PMC article. Review.
-
Why are some antiarrhythmic drugs proarrhythmic? Cardiac arrhythmia study by bifurcation analysis.J Electrocardiol. 1995;28 Suppl:191-7. doi: 10.1016/s0022-0736(95)80055-7. J Electrocardiol. 1995. PMID: 8656110
Cited by
-
Molecular Pathogenesis of Cardiac Arrhythmia.Biomolecules. 2022 Sep 29;12(10):1393. doi: 10.3390/biom12101393. Biomolecules. 2022. PMID: 36291601 Free PMC article.
References
-
- Guckenheimer J.M., Holmes P. Nonlinear Oscillations, Dynamical Systems, and Bifurcations of Vector Fields. Springer; New York, NY, USA: 1986. p. 453.
-
- Nusse H.E., Yorke J.A., Kostelich Y.J. Dynamics: Numerical Explorations. Springer; New York, NY, USA: 1994. p. 484.
-
- Noble D. The Music of Life: Biology beyond the Genome. Oxford University Press; Oxford, UK: 2006. 153p
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

