The Impact of NRF2 Inhibition on Drug-Induced Colon Cancer Cell Death and p53 Activity: A Pilot Study
- PMID: 35327653
- PMCID: PMC8946796
- DOI: 10.3390/biom12030461
The Impact of NRF2 Inhibition on Drug-Induced Colon Cancer Cell Death and p53 Activity: A Pilot Study
Abstract
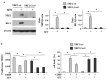
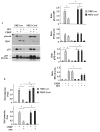
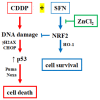
Nuclear factor erythroid 2 (NF-E2) p45-related factor 2 (NRF2) protein is the master regulator of oxidative stress, which is at the basis of various chronic diseases including cancer. Hyperactivation of NRF2 in already established cancers can promote cell proliferation and resistance to therapies, such as in colorectal cancer (CRC), one of the most lethal and prevalent malignancies in industrialized countries with limited patient overall survival due to its escape mechanisms in both chemo- and targeted therapies. In this study, we generated stable NRF2 knockout colon cancer cells (NRF2-Cas9) to investigate the cell response to chemotherapeutic drugs with regard to p53 oncosuppressor, whose inhibition we previously showed to correlate with NRF2 pathway activation. Here, we found that NRF2 activation by sulforaphane (SFN) reduced cisplatin (CDDP)-induced cell death only in NRF2-proficient cells (NRF2-ctr) compared to NRF2-Cas9 cells. Mechanistically, we found that NRF2 activation protected NRF2-ctr cells from the drug-induced DNA damage and the apoptotic function of the unfolded protein response (UPR), in correlation with reduction of p53 activity, effects that were not observed in NRF2-Cas9 cells. Finally, we found that ZnCl2 supplementation rescued the cisplatin cytotoxic effects, as it impaired NRF2 activation, restoring p53 activity. These findings highlight NRF2's key role in neutralizing the cytotoxic effects of chemotherapeutic drugs in correlation with reduced DNA damage and p53 activity. They also suggest that NRF2 inhibition could be a useful strategy for efficient anticancer chemotherapy and support the use of ZnCl2 to inhibit NRF2 pathway in combination therapies.
Keywords: CHOP; DNA damage; NRF2; TP53; ZnCl2 supplementation; apoptosis; colorectal carcinoma; sulforaphane; unfolded protein response (UPR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Nuclear factor erythroid 2 (NF-E2) p45-related factor 2 interferes with homeodomain-interacting protein kinase 2/p53 activity to impair solid tumors chemosensitivity.IUBMB Life. 2020 Aug;72(8):1634-1639. doi: 10.1002/iub.2334. Epub 2020 Jun 27. IUBMB Life. 2020. PMID: 32593231
-
A ruthenium(II)-curcumin compound modulates NRF2 expression balancing the cancer cell death/survival outcome according to p53 status.J Exp Clin Cancer Res. 2020 Jun 30;39(1):122. doi: 10.1186/s13046-020-01628-5. J Exp Clin Cancer Res. 2020. PMID: 32605658 Free PMC article.
-
NRF2 activation in BON‑1 neuroendocrine cancer cells reduces the cytotoxic effects of a novel Ruthenium(II)‑curcumin compound: A pilot study.Oncol Rep. 2024 Feb;51(2):36. doi: 10.3892/or.2024.8695. Epub 2024 Jan 8. Oncol Rep. 2024. PMID: 38186307
-
NRF2 and p53: Januses in cancer?Oncotarget. 2012 Nov;3(11):1272-83. doi: 10.18632/oncotarget.754. Oncotarget. 2012. PMID: 23174755 Free PMC article. Review.
-
Pharmacological Applications of Nrf2 Inhibitors as Potential Antineoplastic Drugs.Int J Mol Sci. 2019 Apr 24;20(8):2025. doi: 10.3390/ijms20082025. Int J Mol Sci. 2019. PMID: 31022969 Free PMC article. Review.
Cited by
-
Screening fructosamine-3-kinase (FN3K) inhibitors, a deglycating enzyme of oncogenic Nrf2: Human FN3K homology modelling, docking and molecular dynamics simulations.PLoS One. 2023 Nov 1;18(11):e0283705. doi: 10.1371/journal.pone.0283705. eCollection 2023. PLoS One. 2023. PMID: 37910519 Free PMC article.
-
HIPK2 as a Novel Regulator of Fibrosis.Cancers (Basel). 2023 Feb 7;15(4):1059. doi: 10.3390/cancers15041059. Cancers (Basel). 2023. PMID: 36831402 Free PMC article. Review.
-
Repurposing of neprilysin inhibitor 'sacubitrilat' as an anti-cancer drug by modulating epigenetic and apoptotic regulators.Sci Rep. 2023 Jun 19;13(1):9952. doi: 10.1038/s41598-023-36872-0. Sci Rep. 2023. PMID: 37336927 Free PMC article.
-
The Sweet Side of HIPK2.Cancers (Basel). 2023 May 9;15(10):2678. doi: 10.3390/cancers15102678. Cancers (Basel). 2023. PMID: 37345014 Free PMC article. Review.
-
Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis.Sci Rep. 2023 Sep 1;13(1):14359. doi: 10.1038/s41598-023-41490-x. Sci Rep. 2023. PMID: 37658132 Free PMC article.
References
-
- Torrente L., Maan G., Rezig A.O., Quinn J., Jackson A., Grilli A., Casares L., Zhang Y., Kulesskiy E., Saarela J., et al. High NRF2 levels correlates with poor prognosis in colorectal cancer patients and sensitivity to the kinase inhibitor AT9283 in vitro. Biomolecules. 2020;10:1365. doi: 10.3390/biom10101365. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

