Alpha-Synuclein-Specific Naturally Occurring Antibodies Inhibit Aggregation In Vitro and In Vivo
- PMID: 35327661
- PMCID: PMC8946620
- DOI: 10.3390/biom12030469
Alpha-Synuclein-Specific Naturally Occurring Antibodies Inhibit Aggregation In Vitro and In Vivo
Abstract
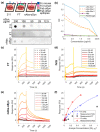
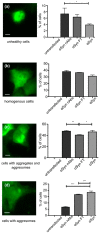
Parkinson's disease (PD) is associated with motor and non-motor symptoms and characterized by aggregates of alpha-synuclein (αSyn). Naturally occurring antibodies (nAbs) are part of the innate immune system, produced without prior contact to their specific antigen, and polyreactive. The abundance of nAbs against αSyn is altered in patients with PD. In this work, we biophysically characterized nAbs against αSyn (nAbs-αSyn) and determined their biological effects. nAbs-αSyn were isolated from commercial intravenous immunoglobulins using column affinity purification. Biophysical properties were characterized using a battery of established in vitro assays. Biological effects were characterized in HEK293T cells transiently transfected with fluorescently tagged αSyn. Specific binding of nAbs-αSyn to monomeric αSyn was demonstrated by Dot blot, ELISA, and Surface Plasmon Resonance. nAbs-αSyn did not affect viability of HEK293T cells as reported by Cell Titer Blue and LDH Assays. nAbs-αSyn inhibited fibrillation of αSyn reported by the Thioflavin T aggregation assay. Altered fibril formation was confirmed with atomic force microscopy. In cells transfected with EGFP-tagged αSyn we observed reduced formation of aggresomes, perinuclear accumulations of αSyn aggregates. The results demonstrate that serum of healthy individuals contains nAbs that specifically bind αSyn and inhibit aggregation of αSyn in vitro. The addition of nAbs-αSyn to cultured cells affects intracellular αSyn aggregates. These findings help understanding the role of the innate immune systems for the pathogenesis of PD and suggest that systemic αSyn binding agents could potentially affect neuronal αSyn pathology.
Keywords: Parkinson’s disease; aggregation; alpha-synuclein; intravenous immunoglobulins (IVIG); naturally occurring antibodies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Alpha-Synuclein Autoimmune Decline in Prodromal Multiple System Atrophy and Parkinson's Disease.Int J Mol Sci. 2022 Jun 12;23(12):6554. doi: 10.3390/ijms23126554. Int J Mol Sci. 2022. PMID: 35742998 Free PMC article.
-
Autoantibodies against α-synuclein inhibit its aggregation and cytotoxicity.J Autoimmun. 2025 Mar;152:103390. doi: 10.1016/j.jaut.2025.103390. Epub 2025 Mar 4. J Autoimmun. 2025. PMID: 40037001
-
Autoimmune antibody decline in Parkinson's disease and Multiple System Atrophy; a step towards immunotherapeutic strategies.Mol Neurodegener. 2017 Jun 7;12(1):44. doi: 10.1186/s13024-017-0187-7. Mol Neurodegener. 2017. PMID: 28592329 Free PMC article.
-
Gangliosides, α-Synuclein, and Parkinson's Disease.Prog Mol Biol Transl Sci. 2018;156:435-454. doi: 10.1016/bs.pmbts.2017.12.009. Epub 2018 Feb 24. Prog Mol Biol Transl Sci. 2018. PMID: 29747823 Review.
-
Pathological role of lipid interaction with α-synuclein in Parkinson's disease.Neurochem Int. 2018 Oct;119:97-106. doi: 10.1016/j.neuint.2017.12.014. Epub 2018 Jan 3. Neurochem Int. 2018. PMID: 29305919 Review.
Cited by
-
Serum Inflammatory Profile in Hereditary Transthyretin Amyloidosis: Mechanisms and Possible Therapeutic Implications.Brain Sci. 2022 Dec 12;12(12):1708. doi: 10.3390/brainsci12121708. Brain Sci. 2022. PMID: 36552168 Free PMC article.
-
Alpha-Synuclein Autoimmune Decline in Prodromal Multiple System Atrophy and Parkinson's Disease.Int J Mol Sci. 2022 Jun 12;23(12):6554. doi: 10.3390/ijms23126554. Int J Mol Sci. 2022. PMID: 35742998 Free PMC article.
-
The immunology of B-1 cells: from development to aging.Immun Ageing. 2024 Aug 2;21(1):54. doi: 10.1186/s12979-024-00455-y. Immun Ageing. 2024. PMID: 39095816 Free PMC article. Review.
-
Nanotechnology meets medicine: applications of atomic force microscopy in disease.Biophys Rev. 2025 Apr 3;17(2):359-384. doi: 10.1007/s12551-025-01306-w. eCollection 2025 Apr. Biophys Rev. 2025. PMID: 40376402 Free PMC article. Review.
-
Human serum-derived α-synuclein auto-antibodies mediate NMDA receptor-dependent degeneration of CNS neurons.J Neuroinflammation. 2024 Feb 28;21(1):62. doi: 10.1186/s12974-024-03050-6. J Neuroinflammation. 2024. PMID: 38419079 Free PMC article.
References
-
- Obeso J.A., Stamelou M., Goetz C.G., Poewe W., Lang A.E., Weintraub D., Burn D., Halliday G.M., Bezard E., Przedborski S., et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017;32:1264–1310. doi: 10.1002/mds.27115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

