G-Quadruplex Matters in Tissue-Specific Tumorigenesis by BRCA1 Deficiency
- PMID: 35327946
- PMCID: PMC8948836
- DOI: 10.3390/genes13030391
G-Quadruplex Matters in Tissue-Specific Tumorigenesis by BRCA1 Deficiency
Abstract
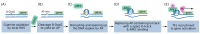
How and why distinct genetic alterations, such as BRCA1 mutation, promote tumorigenesis in certain tissues, but not others, remain an important issue in cancer research. The underlying mechanisms may reveal tissue-specific therapeutic vulnerabilities. Although the roles of BRCA1, such as DNA damage repair and stalled fork stabilization, obviously contribute to tumor suppression, these ubiquitously important functions cannot explain tissue-specific tumorigenesis by BRCA1 mutations. Recent advances in our understanding of the cancer genome and fundamental cellular processes on DNA, such as transcription and DNA replication, have provided new insights regarding BRCA1-associated tumorigenesis, suggesting that G-quadruplex (G4) plays a critical role. In this review, we summarize the importance of G4 structures in mutagenesis of the cancer genome and cell type-specific gene regulation, and discuss a recently revealed molecular mechanism of G4/base excision repair (BER)-mediated transcriptional activation. The latter adequately explains the correlation between the accumulation of unresolved transcriptional regulatory G4s and multi-level genomic alterations observed in BRCA1-associated tumors. In summary, tissue-specific tumorigenesis by BRCA1 deficiency can be explained by cell type-specific levels of transcriptional regulatory G4s and the role of BRCA1 in resolving it. This mechanism would provide an integrated understanding of the initiation and development of BRCA1-associated tumors.
Keywords: BRCA1; BRCAness; G-quadruplex (G4); R-loop; basal-like breast cancer; base excision repair; high-grade serous ovarian carcinoma (HGSC); oxidative genome damage; tissue-specific-tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Impact of G-Quadruplexes on the Regulation of Genome Integrity, DNA Damage and Repair.Biomolecules. 2021 Aug 27;11(9):1284. doi: 10.3390/biom11091284. Biomolecules. 2021. PMID: 34572497 Free PMC article. Review.
-
The Relevance of G-Quadruplexes for DNA Repair.Int J Mol Sci. 2021 Nov 22;22(22):12599. doi: 10.3390/ijms222212599. Int J Mol Sci. 2021. PMID: 34830478 Free PMC article. Review.
-
Deregulated estrogen receptor signaling and DNA damage response in breast tumorigenesis.Biochim Biophys Acta Rev Cancer. 2021 Jan;1875(1):188482. doi: 10.1016/j.bbcan.2020.188482. Epub 2020 Nov 28. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33260050 Review.
-
Differential responses of neurons, astrocytes, and microglia to G-quadruplex stabilization.Aging (Albany NY). 2021 Jun 19;13(12):15917-15941. doi: 10.18632/aging.203222. Epub 2021 Jun 19. Aging (Albany NY). 2021. PMID: 34139671 Free PMC article.
-
Inherited breast cancer: an emerging picture.Clin Genet. 1998 Dec;54(6):447-58. doi: 10.1111/j.1399-0004.1998.tb03764.x. Clin Genet. 1998. PMID: 9894790 Review.
Cited by
-
Mapping the diffusion pattern of 1O2 along DNA duplex by guanine photooxidation with an appended biphenyl photosensitizer.Sci Rep. 2023 Jan 23;13(1):288. doi: 10.1038/s41598-023-27526-2. Sci Rep. 2023. PMID: 36690669 Free PMC article.
-
Epigenomic Features and Potential Functions of K+ and Na+ Favorable DNA G-Quadruplexes in Rice.Int J Mol Sci. 2022 Jul 29;23(15):8404. doi: 10.3390/ijms23158404. Int J Mol Sci. 2022. PMID: 35955535 Free PMC article.
-
Prediction and validation of circulating G-quadruplexes as a novel biomarker in colorectal cancer.J Gastrointest Oncol. 2024 Feb 29;15(1):286-298. doi: 10.21037/jgo-24-26. Epub 2024 Feb 28. J Gastrointest Oncol. 2024. PMID: 38482213 Free PMC article.
-
Increased Age-Adjusted Cancer Mortality After the Third mRNA-Lipid Nanoparticle Vaccine Dose During the COVID-19 Pandemic in Japan.Cureus. 2024 Apr 8;16(4):e57860. doi: 10.7759/cureus.57860. eCollection 2024 Apr. Cureus. 2024. Retraction in: Cureus. 2024 Jun 26;16(6):r143. doi: 10.7759/cureus.r143. PMID: 38721172 Free PMC article. Retracted.
References
-
- Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.-A., Mooij T.M., Roos-Blom M.-J., Jervis S., van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

