Recombination Mediator Proteins: Misnomers That Are Key to Understanding the Genomic Instabilities in Cancer
- PMID: 35327990
- PMCID: PMC8950967
- DOI: 10.3390/genes13030437
Recombination Mediator Proteins: Misnomers That Are Key to Understanding the Genomic Instabilities in Cancer
Abstract
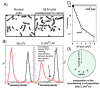
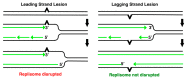
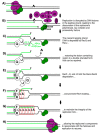
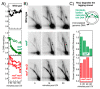
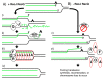
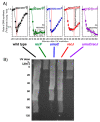
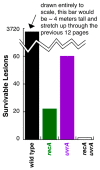
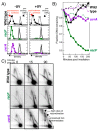
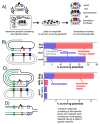
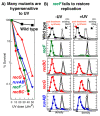
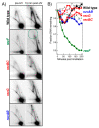
Recombination mediator proteins have come into focus as promising targets for cancer therapy, with synthetic lethal approaches now clinically validated by the efficacy of PARP inhibitors in treating BRCA2 cancers and RECQ inhibitors in treating cancers with microsatellite instabilities. Thus, understanding the cellular role of recombination mediators is critically important, both to improve current therapies and develop new ones that target these pathways. Our mechanistic understanding of BRCA2 and RECQ began in Escherichia coli. Here, we review the cellular roles of RecF and RecQ, often considered functional homologs of these proteins in bacteria. Although these proteins were originally isolated as genes that were required during replication in sexual cell cycles that produce recombinant products, we now know that their function is similarly required during replication in asexual or mitotic-like cell cycles, where recombination is detrimental and generally not observed. Cells mutated in these gene products are unable to protect and process replication forks blocked at DNA damage, resulting in high rates of cell lethality and recombination events that compromise genome integrity during replication.
Keywords: RecF; RecJ; RecO; RecQ; RecR; nucleotide excision repair; recombination; translesion synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
RecO acts with RecF and RecR to protect and maintain replication forks blocked by UV-induced DNA damage in Escherichia coli.J Biol Chem. 2004 Jan 30;279(5):3492-6. doi: 10.1074/jbc.M311012200. Epub 2003 Nov 18. J Biol Chem. 2004. PMID: 14625283
-
Involvement of RecF pathway recombination genes in postreplication repair in UV-irradiated Escherichia coli cells.Mutat Res. 1994 Jul;315(1):1-9. doi: 10.1016/0921-8777(94)90021-3. Mutat Res. 1994. PMID: 7517004
-
Phage lambda has an analog of Escherichia coli recO, recR and recF genes.Genetics. 1992 Jan;130(1):7-16. doi: 10.1093/genetics/130.1.7. Genetics. 1992. PMID: 1310087 Free PMC article.
-
Functions of RecQ family helicases: possible involvement of Bloom's and Werner's syndrome gene products in guarding genome integrity during DNA replication.J Biochem. 2001 Apr;129(4):501-7. doi: 10.1093/oxfordjournals.jbchem.a002883. J Biochem. 2001. PMID: 11275547 Review.
-
Recs preventing wrecks.Mutat Res. 2005 Sep 4;577(1-2):217-27. doi: 10.1016/j.mrfmmm.2005.03.019. Mutat Res. 2005. PMID: 16011837 Review.
Cited by
-
RecF protein targeting to postreplication (daughter strand) gaps I: DNA binding by RecF and RecFR.Nucleic Acids Res. 2023 Jun 23;51(11):5699-5713. doi: 10.1093/nar/gkad311. Nucleic Acids Res. 2023. PMID: 37125642 Free PMC article.
-
Single strand gap repair: The presynaptic phase plays a pivotal role in modulating lesion tolerance pathways.PLoS Genet. 2022 Jun 2;18(6):e1010238. doi: 10.1371/journal.pgen.1010238. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35653392 Free PMC article.
-
F-box DNA Helicase 1 (FBH1) Contributes to the Destabilization of DNA Damage Repair Machinery in Human Cancers.Cancers (Basel). 2023 Sep 6;15(18):4439. doi: 10.3390/cancers15184439. Cancers (Basel). 2023. PMID: 37760409 Free PMC article.
-
The Role of the RecFOR Complex in Genome Stability.Int J Mol Sci. 2025 Jun 6;26(12):5441. doi: 10.3390/ijms26125441. Int J Mol Sci. 2025. PMID: 40564904 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

