Simultaneous Noninvasive Detection and Therapy of Atherosclerosis Using HDL Coated Gold Nanorods
- PMID: 35328130
- PMCID: PMC8947645
- DOI: 10.3390/diagnostics12030577
Simultaneous Noninvasive Detection and Therapy of Atherosclerosis Using HDL Coated Gold Nanorods
Abstract
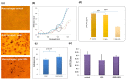
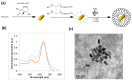
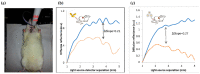
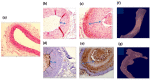
Cardiovascular disease (CVD) is a major cause of death and disability worldwide. A real need exists in the development of new, improved therapeutic methods for treating CVD, while major advances in nanotechnology have opened new avenues in this field. In this paper, we report the use of gold nanoparticles (GNPs) coated with high-density lipoprotein (HDL) (GNP-HDL) for the simultaneous detection and therapy of unstable plaques. Based on the well-known HDL cardiovascular protection, by promoting the reverse cholesterol transport (RCT), injured rat carotids, as a model for unstable plaques, were injected with the GNP-HDL. Noninvasive detection of the plaques 24 h post the GNP injection was enabled using the diffusion reflection (DR) method, indicating that the GNP-HDL particles had accumulated in the injured site. Pathology and noninvasive CT measurements proved the recovery of the injured artery treated with the GNP-HDL. The DR of the GNP-HDL presented a simple and highly sensitive method at a low cost, resulting in simultaneous specific unstable plaque diagnosis and recovery.
Keywords: atherosclerosis; diffusion reflection; gold nanorods; high-density lipoprotein; noninvasive imaging; unstable plaques.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Gold nanorods as absorption contrast agents for the noninvasive detection of arterial vascular disorders based on diffusion reflection measurements.Nano Lett. 2014 May 14;14(5):2681-7. doi: 10.1021/nl500573d. Epub 2014 Apr 8. Nano Lett. 2014. PMID: 24697682
-
Hyperlipidemic mice as a model for a real-time in vivo detection of atherosclerosis by gold nanorods-based diffusion reflection technique.J Biophotonics. 2019 Jan;12(1):e201800218. doi: 10.1002/jbio.201800218. Epub 2018 Oct 11. J Biophotonics. 2019. PMID: 30141260
-
Diffusion Reflection and Fluorescence Lifetime Imaging Microscopy Study of Fluorophore-Conjugated Gold Nanoparticles or Nanorods in Solid Phantoms.ACS Photonics. 2014 Sep 17;1(9):900-905. doi: 10.1021/ph500214m. Epub 2014 Aug 25. ACS Photonics. 2014. PMID: 25541621 Free PMC article.
-
microRNAs in lipoprotein metabolism and cardiometabolic disorders.Atherosclerosis. 2016 Mar;246:352-60. doi: 10.1016/j.atherosclerosis.2016.01.025. Epub 2016 Jan 18. Atherosclerosis. 2016. PMID: 26828754 Free PMC article. Review.
-
HDL and Reverse Cholesterol Transport.Circ Res. 2019 May 10;124(10):1505-1518. doi: 10.1161/CIRCRESAHA.119.312617. Circ Res. 2019. PMID: 31071007 Free PMC article. Review.
Cited by
-
Au nanodyes as enhanced contrast agents in wide field near infrared fluorescence lifetime imaging.Discov Nano. 2024 Jan 25;19(1):18. doi: 10.1186/s11671-024-03958-1. Discov Nano. 2024. PMID: 38270794 Free PMC article.
-
Advances in the treatment of atherosclerotic plaque based on nanomaterials.Nanomedicine (Lond). 2025 Apr;20(8):869-881. doi: 10.1080/17435889.2025.2480049. Epub 2025 Mar 20. Nanomedicine (Lond). 2025. PMID: 40109186 Review.
-
Magnetite-Based Biosensors and Molecular Logic Gates: From Magnetite Synthesis to Application.Biosensors (Basel). 2023 Feb 21;13(3):304. doi: 10.3390/bios13030304. Biosensors (Basel). 2023. PMID: 36979516 Free PMC article. Review.
References
-
- Cannon C.P., Harrington R.A., James S., Ardissino D., Becker R.C., Emanuelsson H., Husted S., Katus H., Keltai M., Khurmi N.S., et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): A randomised double-blind study. Lancet. 2010;375:283–293. doi: 10.1016/S0140-6736(09)62191-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources

