Rapid Diagnostic Test for Hepatitis B Virus Viral Load Based on Recombinase Polymerase Amplification Combined with a Lateral Flow Read-Out
- PMID: 35328174
- PMCID: PMC8946908
- DOI: 10.3390/diagnostics12030621
Rapid Diagnostic Test for Hepatitis B Virus Viral Load Based on Recombinase Polymerase Amplification Combined with a Lateral Flow Read-Out
Abstract
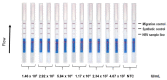
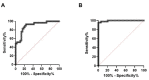
Hepatitis B (HBV) infection is a major public health concern. Perinatal transmission of HBV from mother to child represents the main mode of transmission. Despite the existence of effective immunoprophylaxis, the preventive strategy is inefficient in neonates born to mothers with HBV viral loads above 2 × 105 IU/mL. To prevent mother-to-child transmission, it is important to identify highly viremic pregnant women and initiate antiviral therapy to decrease their viral load. We developed a simple innovative molecular approach avoiding the use of automatic devices to screen highly viremic pregnant women. This method includes rapid DNA extraction coupled with an isothermal recombinase polymerase amplification (RPA) combined with direct visual detection on a lateral flow assay (LFA). We applied our RPA-LFA approach to HBV DNA-positive plasma samples with various loads and genotypes. We designed a triage test by adapting the analytical sensitivity to the recommended therapeutic decision threshold of 2 × 105 IU/mL. The sensitivity and specificity were 98.6% (95% CI: 92.7−99.9%) and 88.2% (95% CI: 73.4−95.3%), respectively. This assay performed excellently, with an area under the ROC curve value of 0.99 (95% CI: 0.99−1.00, p < 0.001). This simple method will open new perspectives in the development of point-of-care testing to prevent HBV perinatal transmission.
Keywords: Chelex extraction; hepatitis B virus; immunochromatographic strip; lateral flow; mother to child transmission; recombinase polymerase amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. [(accessed on 15 July 2021)];2021 Available online: https://www.who.int/publications/i/item/9789240027077.
-
- Stanaway J.D., Flaxman A.D., Naghavi M., Fitzmaurice C., Vos T., Abubakar I., Abu-Raddad L.J., Assadi R., Bhala N., Cowie B., et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

