Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy
- PMID: 35328199
- PMCID: PMC8947468
- DOI: 10.3390/diagnostics12030645
Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy
Abstract
Hypoxic-ischemic encephalopathy (HIE) is a major cause of neurological sequelae in (near-)term newborns. Despite the use of therapeutic hypothermia, a significant number of newborns still experience impaired neurodevelopment. Neuroimaging is the standard of care in infants with HIE to determine the timing and nature of the injury, guide further treatment decisions, and predict neurodevelopmental outcomes. Cranial ultrasonography is a helpful noninvasive tool to assess the brain before initiation of hypothermia to look for abnormalities suggestive of HIE mimics or antenatal onset of injury. Magnetic resonance imaging (MRI) which includes diffusion-weighted imaging has, however, become the gold standard to assess brain injury in infants with HIE, and has an excellent prognostic utility. Magnetic resonance spectroscopy provides complementary metabolic information and has also been shown to be a reliable prognostic biomarker. Advanced imaging modalities, including diffusion tensor imaging and arterial spin labeling, are increasingly being used to gain further information about the etiology and prognosis of brain injury. Over the past decades, tremendous progress has been made in the field of neonatal neuroimaging. In this review, the main brain injury patterns of infants with HIE, the application of conventional and advanced MRI techniques in these newborns, and HIE mimics, will be described.
Keywords: diffusion-weighted imaging; hypoxic-ischemic encephalopathy; magnetic resonance imaging; magnetic resonance spectroscopy; neonatal encephalopathy; neonatal neuroimaging; outcome prediction; perinatal asphyxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
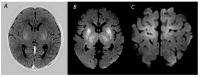
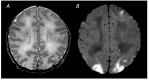
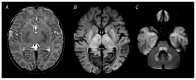
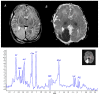
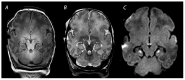
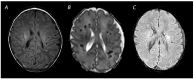
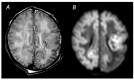
References
-
- Lee A.C., Kozuki N., Blencowe H., Vos T., Bahalim A., Darmstadt G.L., Niermeyer S., Ellis M., Robertson N.J., Cousens S., et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74:50–72. doi: 10.1038/pr.2013.206. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

