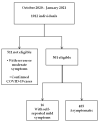
Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients
- PMID: 35328203
- PMCID: PMC8947527
- DOI: 10.3390/diagnostics12030650
Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients
Abstract
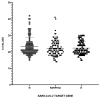
Molecular tests are the gold standard to diagnose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection but are associated with a diagnostic delay, while antigen detection tests can generate results within 20 min even outside a laboratory. In order to evaluate the accuracy and reliability of the FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit (Ag-RDT), two respiratory swabs were collected simultaneously from 501 patients, with mild or no coronavirus disease 2019 (COVID-19)-related symptoms, and analyzed with both the Reverse Transcriptase-quantitative Polymerase Chain Reaction (RT-qPCR) and the FAST COVID-19 SARS-CoV-2 Antigen Rapid Test. Results were then compared to determine clinical performance in a screening setting. We measured a precision of 97.41% (95% CI 92.42-99.15%) and a recall of 98.26% (95% CI 93.88-99.25%), with a specificity of 99.22% (95% CI 97.74-99.74%), a negative predictive value of 99.48% (95% CI 97.98-99.87%), and an overall accuracy of 99.00% (95% CI 97.69-99.68%). Concordance was described by a Kappa coefficient of 0.971 (95% CI 0.947-0.996). Considering short lead times, low cost, and opportunities for decentralized testing, the Ag-RDT test can enhance the efforts to control SARS-CoV-2 spread in several settings.
Keywords: Ag-RDT; COVID-19; RT-qPCR; SARS-CoV-2; antigenic test; diagnosis; surveillance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
-
- World Health Organization Clinical Management of COVID-19: Interim Guidance. 27 May 2020 (who.int) [(accessed on 30 December 2021)]. Available online: https://apps.who.int/iris/handle/10665/332196.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

