Detection of NTRK Fusions and TRK Expression and Performance of pan-TRK Immunohistochemistry in Routine Diagnostics: Results from a Nationwide Community-Based Cohort
- PMID: 35328221
- PMCID: PMC8946871
- DOI: 10.3390/diagnostics12030668
Detection of NTRK Fusions and TRK Expression and Performance of pan-TRK Immunohistochemistry in Routine Diagnostics: Results from a Nationwide Community-Based Cohort
Abstract
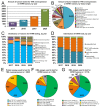
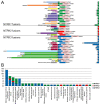
Gene fusions involving NTRK1, NTRK2, and NTRK3 are rare drivers of cancer that can be targeted with histology-agnostic inhibitors. This study aimed to determine the nationwide landscape of NTRK/TRK testing in the Netherlands and the usage of pan-TRK immunohistochemistry (IHC) as a preselection tool to detect NTRK fusions. All pathology reports in 2017-2020 containing the search term 'TRK' were retrieved from the Dutch Pathology Registry (PALGA). Patient characteristics, tumor histology, NTRK/TRK testing methods, and reported results were extracted. NTRK/TRK testing was reported for 7457 tumors. Absolute testing rates increased from 815 (2017) to 3380 (2020). Tumors were tested with DNA/RNA-based molecular assay(s) (48%), IHC (47%), or in combination (5%). A total of 69 fusions involving NTRK1 (n = 22), NTRK2 (n = 6) and NTRK3 (n = 41) were identified in tumors from adult (n = 51) and pediatric (n = 18) patients. In patients tested with both IHC and a molecular assay (n = 327, of which 29 NTRK fusion-positive), pan-TRK IHC had a sensitivity of 77% (95% confidence interval (CI), 56-91) and a specificity of 84% (95% CI, 78-88%). These results showed that pan-TRK IHC has a low sensitivity in current routine practice and warrants the introduction of quality guidelines regarding the implementation and interpretation of pan-TRK IHC.
Keywords: NTRK; TRK; fusion; molecular diagnostics; nationwide; routine; testing.
Conflict of interest statement
H.J.M.G. reports advisory board presence for Bayer, Lilly, Merck, and Novartis, outside the submitted work. W.T. reports consulting fees to the institution from Merck Sharp Dohme, and Bristol-Myers-Squibb, board membership for Dutch Society of Pathology, and the Council for Research and Innovation of the Federation of Medical Specialists, outside the submitted work. E.S. reports providing lectures for Bio-Rad, Novartis, Roche, Biocartis, Illumina, Pfizer, AstraZeneca, and Agena Bioscience, advisory board presence for AstraZeneca, Roche, Pfizer, Novartis, Bayer, Lilly, BMS, Amgen, Biocartis, Illumina, Agena Bioscience, and MSD/Merck, and research grants from Pfizer, Biocartis, Invitae-ArcherDX, AstraZeneca, Agena Bioscience, BMS, Bio-Rad, Roche, Boehringer Ingelheim, all outside the submitted work and all financial supports transferred to Institute. S.M.W. reports unrestricted research grants from Bayer, MSD, Roche, Pfizer, Novartis, Amgen and AstraZeneca, outside the submitted work. L.C.v.K reports grants and non-financial support from Roche, advisory board presence for AstraZeneca, Novartis, Merck, Janssen-Cilag, Bayer, BMS, nanoString and Pfizer, grants and non-financial support from Invitae, non-financial support from Biocartis, grants from Bayer, non-financial support from nanoString, all outside the submitted work. All remaining authors (B.K., C.C.H.J.K.) have declared no conflict of interest.
Figures
Similar articles
-
Pan-tumor screening for NTRK gene fusions using pan-TRK immunohistochemistry and RNA NGS fusion panel testing.Cancer Genet. 2022 Apr;262-263:47-52. doi: 10.1016/j.cancergen.2021.12.010. Epub 2022 Jan 2. Cancer Genet. 2022. PMID: 35007853
-
Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions.Am J Surg Pathol. 2017 Nov;41(11):1547-1551. doi: 10.1097/PAS.0000000000000911. Am J Surg Pathol. 2017. PMID: 28719467 Free PMC article.
-
Detection of NTRK fusions in glioblastoma: fluorescent in situ hybridisation is more useful than pan-TRK immunohistochemistry as a screening tool prior to RNA sequencing.Pathology. 2022 Feb;54(1):55-62. doi: 10.1016/j.pathol.2021.05.100. Epub 2021 Sep 10. Pathology. 2022. PMID: 34518039
-
Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy.J Mol Diagn. 2019 Jul;21(4):553-571. doi: 10.1016/j.jmoldx.2019.03.008. Epub 2019 May 7. J Mol Diagn. 2019. PMID: 31075511 Free PMC article. Review.
-
NTRK Fusions, from the Diagnostic Algorithm to Innovative Treatment in the Era of Precision Medicine.Int J Mol Sci. 2020 May 25;21(10):3718. doi: 10.3390/ijms21103718. Int J Mol Sci. 2020. PMID: 32466202 Free PMC article. Review.
Cited by
-
NTRK Fusions in 1113 Solid Tumors in a Single Institution.Diagnostics (Basel). 2022 Jun 13;12(6):1450. doi: 10.3390/diagnostics12061450. Diagnostics (Basel). 2022. PMID: 35741260 Free PMC article.
-
Cost-Efficient Detection of NTRK1/2/3 Gene Fusions: Single-Center Analysis of 8075 Tumor Samples.Int J Mol Sci. 2023 Sep 17;24(18):14203. doi: 10.3390/ijms241814203. Int J Mol Sci. 2023. PMID: 37762506 Free PMC article.
-
Limited Accuracy of Pan-Trk Immunohistochemistry Screening for NTRK Rearrangements in Follicular-Derived Thyroid Carcinoma.Int J Mol Sci. 2022 Jul 5;23(13):7470. doi: 10.3390/ijms23137470. Int J Mol Sci. 2022. PMID: 35806472 Free PMC article.
-
Analysis of CD74 Occurrence in Oncogenic Fusion Proteins.Int J Mol Sci. 2023 Nov 5;24(21):15981. doi: 10.3390/ijms242115981. Int J Mol Sci. 2023. PMID: 37958963 Free PMC article. Review.
-
Comparison of NTRK fusion detection methods in microsatellite-instability-high metastatic colorectal cancer.Virchows Arch. 2023 Jun;482(6):983-992. doi: 10.1007/s00428-023-03538-1. Epub 2023 Apr 17. Virchows Arch. 2023. PMID: 37067589 Free PMC article.
References
-
- Skálová A., Vanecek T., Sima R., Laco J., Weinreb I., Perez-Ordonez B., Starek I., Geierova M., Simpson R.H.W., Passador-Santos F., et al. Mammary Analogue Secretory Carcinoma of Salivary Glands, Containing the ETV6-NTRK3 Fusion Gene: A Hitherto Undescribed Salivary Gland Tumor Entity. Am. J. Surg. Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. - DOI - PubMed
-
- Tognon C., Knezevich S.R., Huntsman D., Roskelley C.D., Melnyk N., Mathers J.A., Becker L., Carneiro F., MacPherson N., Horsman D., et al. Expression of the ETV6-NTRK3 Gene Fusion as a Primary Event in Human Secretory Breast Carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. - DOI - PubMed
-
- Rubin B.P., Chen C.J., Morgan T.W., Xiao S., Grier H.E., Kozakewich H.P., Perez-Atayde A.R., Fletcher J.A. Congenital Mesoblastic Nephroma t(12;15) Is Associated with ETV6-NTRK3 Gene Fusion: Cytogenetic and Molecular Relationship to Congenital (Infantile) Fibrosarcoma. Am. J. Pathol. 1998;153:1451–1458. doi: 10.1016/S0002-9440(10)65732-X. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

