GLUT3/SLC2A3 Is an Endogenous Marker of Hypoxia in Prostate Cancer Cell Lines and Patient-Derived Xenograft Tumors
- PMID: 35328229
- PMCID: PMC8946944
- DOI: 10.3390/diagnostics12030676
GLUT3/SLC2A3 Is an Endogenous Marker of Hypoxia in Prostate Cancer Cell Lines and Patient-Derived Xenograft Tumors
Abstract
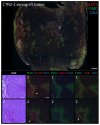
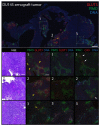
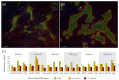
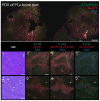
The microenvironment of solid tumors is dynamic and frequently contains pockets of low oxygen levels (hypoxia) surrounded by oxygenated tissue. Indeed, a compromised vasculature is a hallmark of the tumor microenvironment, creating both spatial gradients and temporal variability in oxygen availability. Notably, hypoxia associates with increased metastasis and poor survival in patients. Therefore, to aid therapeutic decisions and better understand hypoxia's role in cancer progression, it is critical to identify endogenous biomarkers of hypoxia to spatially phenotype oncogenic lesions in human tissue, whether precancerous, benign, or malignant. Here, we characterize the glucose transporter GLUT3/SLC2A3 as a biomarker of hypoxic prostate epithelial cells and prostate tumors. Transcriptomic analyses of non-tumorigenic, immortalized prostate epithelial cells revealed a highly significant increase in GLUT3 expression under hypoxia. Additionally, GLUT3 protein increased 2.4-fold in cultured hypoxic prostate cell lines and was upregulated within hypoxic regions of xenograft tumors, including two patient-derived xenografts (PDX). Finally, GLUT3 out-performs other established hypoxia markers; GLUT3 staining in PDX specimens detects 2.6-8.3 times more tumor area compared to a mixture of GLUT1 and CA9 antibodies. Therefore, given the heterogeneous nature of tumors, we propose adding GLUT3 to immunostaining panels when trying to detect hypoxic regions in prostate samples.
Keywords: GLUT3; SLC2A3; glucose transporter; hypoxia; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Expression of hypoxia-related glucose transporters GLUT1 and GLUT3 in benign, malignant and non-neoplastic thyroid lesions.Mol Med Rep. 2012 Sep;6(3):601-6. doi: 10.3892/mmr.2012.969. Epub 2012 Jun 27. Mol Med Rep. 2012. PMID: 22752218
-
Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia.Gastroenterology. 2019 Sep;157(3):823-837. doi: 10.1053/j.gastro.2019.05.004. Epub 2019 May 9. Gastroenterology. 2019. PMID: 31078621
-
GLUT3 expression in cystic change induced by hypoxia in pituitary adenomas.Endocr Connect. 2018 Dec;7(12):1518-1527. doi: 10.1530/EC-18-0444. Endocr Connect. 2018. PMID: 30521480 Free PMC article.
-
Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer.J Cell Physiol. 2005 Mar;202(3):654-62. doi: 10.1002/jcp.20166. J Cell Physiol. 2005. PMID: 15389572 Review.
-
Cellular effects and clinical implications of SLC2A3 copy number variation.J Cell Physiol. 2020 Dec;235(12):9021-9036. doi: 10.1002/jcp.29753. Epub 2020 May 5. J Cell Physiol. 2020. PMID: 32372501 Review.
Cited by
-
Physiomimetic Fluidic Culture Platform on Microwell-Patterned Porous Collagen Scaffold for Human Pancreatic Islets.Cell Transplant. 2024 Jan-Dec;33:9636897241249556. doi: 10.1177/09636897241249556. Cell Transplant. 2024. PMID: 38742734 Free PMC article.
-
Identification of SLC2A3 as a prognostic indicator correlated with the NF-κB/EMT axis and immune response in head and neck squamous cell carcinoma.Channels (Austin). 2023 Dec;17(1):2208928. doi: 10.1080/19336950.2023.2208928. Channels (Austin). 2023. PMID: 37134043 Free PMC article.
-
The predictive accuracy of preoperative erythrocyte count and maximum tumor diameter to maximum kidney diameter ratio in renal cell carcinoma.Transl Androl Urol. 2022 Jul;11(7):974-981. doi: 10.21037/tau-22-414. Transl Androl Urol. 2022. PMID: 35958894 Free PMC article.
-
New Therapeutic Perspectives in Prostate Cancer: Patient-Derived Organoids and Patient-Derived Xenograft Models in Precision Medicine.Biomedicines. 2023 Oct 10;11(10):2743. doi: 10.3390/biomedicines11102743. Biomedicines. 2023. PMID: 37893116 Free PMC article. Review.
-
Druggable Metabolic Vulnerabilities Are Exposed and Masked during Progression to Castration Resistant Prostate Cancer.Biomolecules. 2022 Oct 28;12(11):1590. doi: 10.3390/biom12111590. Biomolecules. 2022. PMID: 36358940 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

