Commercially Available Heart Rate Monitor Repurposed for Automatic Arrhythmia Detection with Snapshot Electrocardiographic Capability: A Pilot Validation
- PMID: 35328265
- PMCID: PMC8947007
- DOI: 10.3390/diagnostics12030712
Commercially Available Heart Rate Monitor Repurposed for Automatic Arrhythmia Detection with Snapshot Electrocardiographic Capability: A Pilot Validation
Abstract
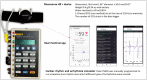
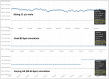
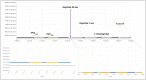
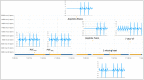
The usefulness of opportunistic arrhythmia screening strategies, using an electrocardiogram (ECG) or other methods for random "snapshot" assessments is limited by the unexpected and occasional nature of arrhythmias, leading to a high rate of missed diagnosis. We have previously validated a cardiac monitoring system for AF detection pairing simple consumer-grade Bluetooth low-energy (BLE) heart rate (HR) sensors with a smartphone application (RITMIA™, Heart Sentinel srl, Italy). In the current study, we test a significant upgrade to the above-mentioned system, thanks to the technical capability of new HR sensors to run algorithms on the sensor itself and to acquire, and store on-board, single-lead ECG strips. We have reprogrammed an HR monitor intended for sports use (Movensense HR+) to run our proprietary RITMIA algorithm code in real-time, based on RR analysis, so that if any type of arrhythmia is detected, it triggers a brief retrospective recording of a single-lead ECG, providing tracings of the specific arrhythmia for later consultation. We report the initial data on the behavior, feasibility, and high diagnostic accuracy of this ultra-low weight customized device for standalone automatic arrhythmia detection and ECG recording, when several types of arrhythmias were simulated under different baseline conditions. Conclusions: The customized device was capable of detecting all types of simulated arrhythmias and correctly triggered a visually interpretable ECG tracing. Future human studies are needed to address real-life accuracy of this device.
Keywords: arrhythmia; cardiac monitoring; cardiovascular prevention; digital cardiology; electrocardiogram; heart failure; sensors; sports cardiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Noseworthy P.A., Kaufman E.S., Chen L.Y., Chung M.K., Elkind M.S., Joglar J.A., Leal M.A., McCabe P.J., Pokorney S.D., Yao X. Subclinical and Device-Detected Atrial Fibrillation: Pondering the Knowledge Gap: A Scientific Statement From the American Heart Association. Circulation. 2019;140:e944–e963. doi: 10.1161/CIR.0000000000000740. - DOI - PMC - PubMed
-
- Svendsen J.H., Diederichsen S.Z., Højberg S., Krieger D.W., Graff C., Kronborg C., Olesen M.S., Nielsen J.B., Holst A.G., Brandes A., et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet. 2021;398:1507–1516. doi: 10.1016/S0140-6736(21)01698-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

