Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas
- PMID: 35328286
- PMCID: PMC8946957
- DOI: 10.3390/diagnostics12030733
Clinicopathological Assessment of Cancer/Testis Antigens NY-ESO-1 and MAGE-A4 in Highly Aggressive Soft Tissue Sarcomas
Abstract
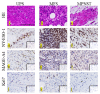
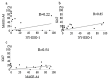
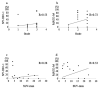
We aimed to investigate the clinical significance of the expression of NY-ESO-1 and MAGE-A4 in soft tissue sarcoma (STS). Immunostaining for NY-ESO-1, MAGE-A4, and Ki67 was performed using pathological specimens harvested from 10 undifferentiated pleomorphic sarcoma (UPS), nine myxofibrosarcoma (MFS), and three malignant peripheral nerve sheath tumor (MPNST) patients treated at our hospital. We examined the correlation of NY-ESO-1 and MAGE-A4 expression levels with tumor size, histological grade, and SUVmax values. Positive cell rates of various markers were also compared between patients in remission and those who were not in remission. The rates of cases positive for NY-ESO, MAGE-A4, and Ki67 were 50%, 63.6%, and 90.9%, respectively. The average rates of cells positive for NY-ESO, MAGE-A4, and Ki67 in all STS types were 18.2%, 39.4%, and 16.8%, respectively. A positive correlation was observed between rates of cells positive for NY-ESO-1 and MAGE-A4 and between NY-ESO-1 and MAGE-A4 expression levels and clinical features. There was no significant difference in the positive cell rate of NY-ESO-1 or MAGE-A4 between remission and non-remission cases. Our results suggest that NY-ESO-1 and MAGE-A4 expression may be useful for the diagnosis and prognostication of UPS, MFS, and MPNST.
Keywords: MAGE-A4; NY-ESO-1; antigens; soft tissue sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fletcher C.D., Lazar A.J., Baldini E.H., Messiou C., Blay J.Y., Pollock R.E., Gronchi A., Singer S. Soft Tissue and Bone Tumours. In: Antonescu C.R., editor. WHO Classification of Tumors. 5th ed. Volume 5. IARC Publications; Lyon, France: 2020. pp. 6–12.
-
- Penel N., Coindre J.M., Giraud A., Terrier P., Ranchere-Vince D., Collin F., Guellec S.L.E., Bazille C., Lae M., de Pinieux G., et al. Presentation and outcome of frequent and rare sarcoma histologic subtypes: A study of 10,262 patients with localized visceral/soft tissue sarcoma managed in reference centers. Cancer. 2018;124:1179–1187. doi: 10.1002/cncr.31176. - DOI - PubMed
-
- Tanaka K., Mizusawa J., Fukuda H., Araki N., Chuman H., Takahashi M., Ozaki T., Hiruma T., Tsuchiya H., Morioka H., et al. Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcomas in the extremities (JCOG0304) Jpn. J. Clin. Oncol. 2015;45:555–561. doi: 10.1093/jjco/hyv042. - DOI - PubMed
-
- Davis E.J., Chugh R., Zhao L., Lucas D.R., Biermann J.S., Zalupski M.M., Feng M., Wong S.L., Jacobson J., Zyczynski L., et al. A randomized open-label phase II study of neo/adjuvant doxorubicin and ifosfamide versus gemcidabine and docetaxel in patients with localized, high risk, soft tissue sarcoma. Eur. J. Cancer. 2015;51:1794–1802. doi: 10.1016/j.ejca.2015.05.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

