Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo
- PMID: 35328299
- PMCID: PMC8947034
- DOI: 10.3390/diagnostics12030746
Field Experiences with Handheld Diagnostic Devices to Triage Children under Five Presenting with Severe Febrile Illness in a District Hospital in DR Congo
Abstract
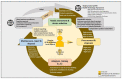
As part of a field study (NCT04473768) in children presenting with severe febrile illness to Kisantu hospital (DR Congo), we retrospectively compiled user experiences (not performance) with handheld diagnostic devices assisting triage: tympanic thermometer, pulse oximeter (measuring heart rate, respiratory rate and oxygen saturation), hemoglobinometer and glucometer. Guidance documents for product selection were generic and scattered. Stock rupture, market withdrawal and unaffordable prices interfered with procurement. Challenges at implementation included environmental temperature, capillary blood sampling (antisepsis, order of multiple tests, filling microcuvettes and glucose strips), calibration (environmental temperature, cold chain) and liability-oriented communication with a manufacturer. Instructions for use were readable and contained symbol keys; two devices had printed French-language instructions. Shortcomings were poor integration of figures with text and distinct procedures for the oximeter and its sensor. Usability interview revealed appreciations for quick results, visibility of the display and memory function (three devices) but also problems of capillary blood sample transfer, cleaning, too long of a time-to-results (respiratory rate) and size, fitting and disposal of thermometer probes. Pictorial error messages were preferred over alphanumeric error codes but interpretation of symbols was poor. Alarm sounds of the oximeter caused unrest in children and caretakers perceived the device as associated with poor prognosis.
Keywords: children under five; danger sign; handheld diagnostic device; instructions for use; label comprehension; low-resource setting; severe febrile illness; triage; usability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Performance of Automated Point-of-Care Respiratory Rate Counting versus Manual Counting in Children under Five Admitted with Severe Febrile Illness to Kisantu Hospital, DR Congo.Diagnostics (Basel). 2021 Nov 10;11(11):2078. doi: 10.3390/diagnostics11112078. Diagnostics (Basel). 2021. PMID: 34829427 Free PMC article.
-
User Perceptions and Experiences of a Handheld 12-Lead Electrocardiographic Device in a Clinical Setting: Usability Evaluation.JMIR Cardio. 2021 Aug 26;5(2):e21186. doi: 10.2196/21186. JMIR Cardio. 2021. PMID: 34435958 Free PMC article.
-
Comparison of the Effects of Motion and Environment Conditions on Accuracy of Handheld and Finger-Based Pulse Oximeters.Mil Med. 2021 Jan 25;186(Suppl 1):465-472. doi: 10.1093/milmed/usaa314. Mil Med. 2021. PMID: 33499470
-
Electronic physiologic and subjective data acquisition in home-dwelling heart failure patients: An assessment of patient use and perception of usability.Int J Med Inform. 2016 Sep;93:42-8. doi: 10.1016/j.ijmedinf.2016.06.001. Epub 2016 Jun 3. Int J Med Inform. 2016. PMID: 27435946
-
Diagnostic accuracy of handheld electrocardiogram devices in detecting atrial fibrillation in adults in community versus hospital settings: a systematic review and meta-analysis.Heart. 2020 Aug;106(16):1211-1217. doi: 10.1136/heartjnl-2020-316611. Epub 2020 May 11. Heart. 2020. PMID: 32393588
Cited by
-
Use of point-of-care haemoglobin tests to diagnose childhood anaemia in low- and middle-income countries: A systematic review.Trop Med Int Health. 2024 Feb;29(2):73-87. doi: 10.1111/tmi.13957. Epub 2023 Dec 3. Trop Med Int Health. 2024. PMID: 38044262 Free PMC article.
-
Nontyphoidal Salmonella Invasive Disease: Challenges and Solutions.Open Forum Infect Dis. 2023 Jun 2;10(Suppl 1):S32-S37. doi: 10.1093/ofid/ofad020. eCollection 2023 May. Open Forum Infect Dis. 2023. PMID: 37274526 Free PMC article.
References
-
- Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, Regional, and National Causes of under-5 Mortality in 2000–15: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. - DOI - PMC - PubMed
-
- Daily J. Fever Diagnostic Technology Landscape. World Health Organization; Geneva, Switzerland: 2018.
-
- Roddy P., Dalrymple U., Jensen Id T.O., Dittrich S., Rao V.B., Pfeffer D.A., Twohig K.A., Roberts T., Bernal O., Guillen E. Quantifying the Incidence of Severe-Febrile-Illness Hospital Admissions in Sub-Saharan Africa. PLoS ONE. 2019;14:e022037. doi: 10.1371/journal.pone.0220371. - DOI - PMC - PubMed
-
- World Health Organization . In: Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. 2nd ed. World Health Organization, editor. World Health Organization; Geneva, Switzerland: 2013. - PubMed
Grants and funding
- Framework Agreement between the Belgian DGD and the Institute of Tropical Medicine/Belgian Directorate of Development Cooperation (DGD)
- 1153220N/Research Foundation - Flanders
- 815439/European Union's Horizon 2020 research and innovation program under the Vacc-iNTS project
- Research Grant: Towards evidence-based treatment of children with non-typhoidal Salmonella bloodstream infection in sub-Saharan Africa/European Society of Clinical Microbiology and Infectious Diseases
LinkOut - more resources
Full Text Sources
Research Materials

