Liquid Profiling for Cancer Patient Stratification in Precision Medicine-Current Status and Challenges for Successful Implementation in Standard Care
- PMID: 35328301
- PMCID: PMC8947441
- DOI: 10.3390/diagnostics12030748
Liquid Profiling for Cancer Patient Stratification in Precision Medicine-Current Status and Challenges for Successful Implementation in Standard Care
Abstract
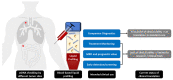
Circulating tumor DNA (ctDNA), accurately described by the term liquid profiling (LP), enables real-time assessment of the tumor mutational profile as a minimally invasive test and has therefore rapidly gained traction, particular for the management of cancer patients. By LP, tumor-specific genetic alterations can be determined as part of companion diagnostics to guide selection of appropriate targeted therapeutics. Because LP facilitates longitudinal monitoring of cancer patients, it can be used to detect acquired resistant mechanisms or as a personalized biomarker for earlier detection of disease recurrence, among other applications. However, LP is not yet integrated into routine care to the extent that might be expected. This is due to the lack of harmonization and standardization of preanalytical and analytical workflows, the lack of proper quality controls, limited evidence of its clinical utility, heterogeneous study results, the uncertainty of clinicians regarding the value and appropriate indications for LP and its interpretation, and finally, the lack of reimbursement for most LP tests. In this review, the value proposition of LP for cancer patient management and treatment optimization, the current status of implementation in standard care, and the main challenges that need to be overcome are discussed in detail.
Keywords: cancer management; cell-free DNA; circulating tumor DNA; clinical oncology; liquid biopsy; liquid profiling; personalized medicine; standard care.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Circulating Tumor DNA in Pediatric Cancer.Front Mol Biosci. 2022 May 12;9:885597. doi: 10.3389/fmolb.2022.885597. eCollection 2022. Front Mol Biosci. 2022. PMID: 35647029 Free PMC article. Review.
-
Current Perspectives on Circulating Tumor DNA, Precision Medicine, and Personalized Clinical Management of Cancer.Mol Cancer Res. 2020 Apr;18(4):517-528. doi: 10.1158/1541-7786.MCR-19-0768. Epub 2020 Jan 29. Mol Cancer Res. 2020. PMID: 31996469 Review.
-
Using circulating cell-free DNA to monitor personalized cancer therapy.Crit Rev Clin Lab Sci. 2017 May;54(3):205-218. doi: 10.1080/10408363.2017.1299683. Epub 2017 Apr 10. Crit Rev Clin Lab Sci. 2017. PMID: 28393575 Review.
-
Molecular tests and target therapies in oncology: recommendations from the Italian workshop.Future Oncol. 2021 Sep;17(26):3529-3539. doi: 10.2217/fon-2021-0286. Epub 2021 Jul 13. Future Oncol. 2021. PMID: 34254524
-
Liquid Biopsy in Colorectal Cancer: Quo Vadis? Implementation of Liquid Biopsies in Routine Clinical Patient Care in Two German Comprehensive Cancer Centers.Front Oncol. 2022 May 12;12:870411. doi: 10.3389/fonc.2022.870411. eCollection 2022. Front Oncol. 2022. PMID: 35646657 Free PMC article.
Cited by
-
Defining the value proposition in diagnostic technology: challenges and opportunities for its understanding and development - a review with a multiperspective reflective analysis.Front Med (Lausanne). 2025 Feb 20;12:1498618. doi: 10.3389/fmed.2025.1498618. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40051729 Free PMC article. Review.
-
Clinical Significance of Clonal Hematopoiesis of Indeterminate Potential in Hematology and Cardiovascular Disease.Diagnostics (Basel). 2022 Jul 2;12(7):1613. doi: 10.3390/diagnostics12071613. Diagnostics (Basel). 2022. PMID: 35885518 Free PMC article. Review.
-
Skin Cancer Research Goes Digital: Looking for Biomarkers within the Droplets.J Pers Med. 2022 Jul 13;12(7):1136. doi: 10.3390/jpm12071136. J Pers Med. 2022. PMID: 35887633 Free PMC article. Review.
-
Point of Care Liquid Biopsy for Cancer Treatment-Early Experience from a Community Center.Cancers (Basel). 2024 Jul 10;16(14):2505. doi: 10.3390/cancers16142505. Cancers (Basel). 2024. PMID: 39061145 Free PMC article.
-
The Potential and Emerging Role of Quantitative Imaging Biomarkers for Cancer Characterization.Cancers (Basel). 2022 Jul 9;14(14):3349. doi: 10.3390/cancers14143349. Cancers (Basel). 2022. PMID: 35884409 Free PMC article. Review.
References
-
- Alix-Panabières C., Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858–873. doi: 10.1158/2159-8290.CD-20-1311. - DOI - PubMed
-
- Aggarwal C., Thompson J.C., Black T.A., Katz S.I., Fan R., Yee S.S., Chien A.L., Evans T.L., Bauml J.M., Alley E.W., et al. Clinical Implications of Plasma-Based Genotyping With the Delivery of Personalized Therapy in Metastatic Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5:173–180. doi: 10.1001/jamaoncol.2018.4305. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources