The Utility of Endoscopic-Ultrasonography-Guided Tissue Acquisition for Solid Pancreatic Lesions
- PMID: 35328306
- PMCID: PMC8947755
- DOI: 10.3390/diagnostics12030753
The Utility of Endoscopic-Ultrasonography-Guided Tissue Acquisition for Solid Pancreatic Lesions
Abstract
Endoscopic-ultrasonography-guided tissue acquisition (EUS-TA) has been widely performed for the definitive diagnosis of solid pancreatic lesions (SPLs). As the puncture needles, puncture techniques, and sample processing methods have improved, EUS-TA has shown higher diagnostic yields and safety. Recently, several therapeutic target genomic biomarkers have been clarified in pancreatic ductal carcinoma (PDAC). Although only a small proportion of patients with PDAC can benefit from precision medicine based on gene mutations at present, precision medicine will also be further developed for SPLs as more therapeutic target genomic biomarkers are identified. Advances in next-generation sequencing (NGS) techniques enable the examination of multiple genetic mutations in limited tissue samples. EUS-TA is also useful for NGS and will play a more important role in determining treatment strategies. In this review, we describe the utility of EUS-TA for SPLs.
Keywords: EUS-guided fine-needle aspiration; EUS-guided fine-needle biopsy; EUS-guided tissue acquisition; endoscopic ultrasonography; pancreatic ductal adenocarcinoma; pancreatic neuroendocrine neoplasms; solid pancreatic lesions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

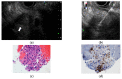

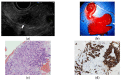
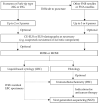
References
-
- Yoshida N., Kanno A., Masamune A., Nabeshima T., Hongo S., Miura S., Takikawa T., Hamada S., Kikuta K., Kume K., et al. Pancreatic Acinar Cell Carcinoma with Multiple Liver Metastases Effectively Treated by S-1 Chemotherapy. Intern. Med. 2018;57:3529–3535. doi: 10.2169/internalmedicine.0294-17. - DOI - PMC - PubMed
-
- Oka K., Inoue K., Sugino S., Harada T., Tsuji T., Nakashima S., Katayama T., Okuda T., Kin S., Nagata A., et al. Anaplastic carcinoma of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: A case report and review of the literature. J. Med. Case Rep. 2018;12:152. doi: 10.1186/s13256-018-1615-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

