Intraocular Delivery of a Collagen Mimetic Peptide Repairs Retinal Ganglion Cell Axons in Chronic and Acute Injury Models
- PMID: 35328332
- PMCID: PMC8949359
- DOI: 10.3390/ijms23062911
Intraocular Delivery of a Collagen Mimetic Peptide Repairs Retinal Ganglion Cell Axons in Chronic and Acute Injury Models
Abstract
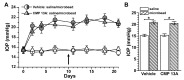
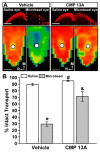
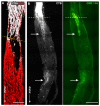
Vision loss through the degeneration of retinal ganglion cell (RGC) axons occurs in both chronic and acute conditions that target the optic nerve. These include glaucoma, in which sensitivity to intraocular pressure (IOP) causes early RGC axonal dysfunction, and optic nerve trauma, which causes rapid axon degeneration from the site of injury. In each case, degeneration is irreversible, necessitating new therapeutics that protect, repair, and regenerate RGC axons. Recently, we demonstrated the reparative capacity of using collagen mimetic peptides (CMPs) to heal fragmented collagen in the neuronal extracellular milieu. This was an important step in the development of neuronal-based therapies since neurodegeneration involves matrix metalloproteinase (MMP)-mediated remodeling of the collagen-rich environment in which neurons and their axons exist. We found that intraocular delivery of a CMP comprising single-strand fractions of triple helix human type I collagen prevented early RGC axon dysfunction in an inducible glaucoma model. Additionally, CMPs also promoted neurite outgrowth from dorsal root ganglia, challenged in vitro by partial digestion of collagen. Here, we compared the ability of a CMP sequence to protect RGC axons in both inducible glaucoma and optic nerve crush. A three-week +40% elevation in IOP caused a 67% degradation in anterograde transport to the superior colliculus, the primary retinal projection target in rodents. We found that a single intravitreal injection of CMP during the period of IOP elevation significantly reduced this degradation. The same CMP delivered shortly after optic nerve crush promoted significant axonal recovery during the two-week period following injury. Together, these findings support a novel protective and reparative role for the use of CMPs in both chronic and acute conditions affecting the survival of RGC axons in the optic projection to the brain.
Keywords: collagen mimetic peptides; collagen reparative; extracellular matrix; glaucoma; neuroprotection; optic nerve crush; optic neuropathy.
Conflict of interest statement
Authors R.O.B, B.J.D.B., and E.S. are employed by Stuart Therapeutics, Inc. Author D.J.C. has received research grants from Stuart Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Restoring the Extracellular Matrix: A Neuroprotective Role for Collagen Mimetic Peptides in Experimental Glaucoma.Front Pharmacol. 2021 Nov 2;12:764709. doi: 10.3389/fphar.2021.764709. eCollection 2021. Front Pharmacol. 2021. PMID: 34795592 Free PMC article.
-
The dark phase intraocular pressure elevation and retinal ganglion cell degeneration in a rat model of experimental glaucoma.Exp Eye Res. 2013 Jul;112:21-8. doi: 10.1016/j.exer.2013.04.008. Epub 2013 Apr 18. Exp Eye Res. 2013. PMID: 23603611 Free PMC article.
-
Bax Contributes to Retinal Ganglion Cell Dendritic Degeneration During Glaucoma.Mol Neurobiol. 2022 Mar;59(3):1366-1380. doi: 10.1007/s12035-021-02675-5. Epub 2022 Jan 5. Mol Neurobiol. 2022. PMID: 34984584 Free PMC article.
-
Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss.Exp Eye Res. 2015 Dec;141:111-24. doi: 10.1016/j.exer.2015.06.006. Epub 2015 Jun 9. Exp Eye Res. 2015. PMID: 26070986 Review.
-
Adaptive responses to neurodegenerative stress in glaucoma.Prog Retin Eye Res. 2021 Sep;84:100953. doi: 10.1016/j.preteyeres.2021.100953. Epub 2021 Feb 25. Prog Retin Eye Res. 2021. PMID: 33640464 Free PMC article. Review.
Cited by
-
Collagen Mimetic Peptides Promote Adherence and Migration of ARPE-19 Cells While Reducing Inflammatory and Oxidative Stress.Int J Mol Sci. 2022 Jun 23;23(13):7004. doi: 10.3390/ijms23137004. Int J Mol Sci. 2022. PMID: 35806007 Free PMC article.
-
Extracellular-Matrix Mechanics Regulate the Ocular Physiological and Pathological Activities.J Ophthalmol. 2023 Jul 22;2023:7626920. doi: 10.1155/2023/7626920. eCollection 2023. J Ophthalmol. 2023. PMID: 37521908 Free PMC article. Review.
-
Collagen mimetic peptides as novel therapeutics for vascular disease in the central nervous system.Front Neurosci. 2025 May 12;19:1569347. doi: 10.3389/fnins.2025.1569347. eCollection 2025. Front Neurosci. 2025. PMID: 40421131 Free PMC article.
-
Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress.Pharmaceuticals (Basel). 2025 Jun 12;18(6):875. doi: 10.3390/ph18060875. Pharmaceuticals (Basel). 2025. PMID: 40573271 Free PMC article.
-
A Phase 2 Trial to Test Safety and Efficacy of ST-100, a Unique Collagen Mimetic Peptide Ophthalmic Solution for Dry Eye Disease.Ophthalmol Sci. 2023 Dec 12;4(3):100451. doi: 10.1016/j.xops.2023.100451. eCollection 2024 May-Jun. Ophthalmol Sci. 2023. PMID: 38317866 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

