Sodium-Iodate Injection Can Replicate Retinal Degenerative Disease Stages in Pigmented Mice and Rats: Non-Invasive Follow-Up Using OCT and ERG
- PMID: 35328338
- PMCID: PMC8953416
- DOI: 10.3390/ijms23062918
Sodium-Iodate Injection Can Replicate Retinal Degenerative Disease Stages in Pigmented Mice and Rats: Non-Invasive Follow-Up Using OCT and ERG
Abstract
Purpose: The lack of suitable animal models for (dry) age-related macular degeneration (AMD) has hampered therapeutic research into the disease, so far. In this study, pigmented rats and mice were systematically injected with various doses of sodium iodate (SI). After injection, the retinal structure and visual function were non-invasively characterized over time to obtain in-depth data on the suitability of these models for studying experimental therapies for retinal degenerative diseases, such as dry AMD.
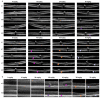
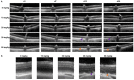
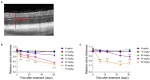
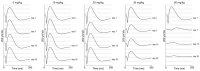
Methods: SI was injected into the tail vein (i.v.) using a series of doses (0-70 mg/kg) in adolescent C57BL/6J mice and Brown Norway rats. The retinal structure and function were assessed non-invasively at baseline (day 1) and at several time points (1-3, 5, and 10-weeks) post-injection by scanning laser ophthalmoscopy (SLO), optical coherence tomography (OCT), and electroretinography (ERG).
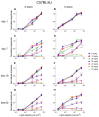
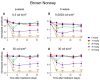
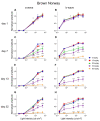
Results: After the SI injection, retinal degeneration in mice and rats yielded similar results. The lowest dose (10 mg/kg) resulted in non-detectable structural or functional effects. An injection with 20 mg/kg SI did not result in an evident retinal degeneration as judged from the OCT data. In contrast, the ERG responses were temporarily decreased but returned to baseline within two-weeks. Higher doses (30, 40, 50, and 70 mg/kg) resulted in moderate to severe structural RPE and retinal injury and decreased the ERG amplitudes, indicating visual impairment in both mice and rat strains.
Conclusions: After the SI injections, we observed dose-dependent structural and functional pathological effects on the retinal pigment epithelium (RPE) and retina in the pigmented mouse and rat strains that were used in this study. Similar effects were observed in both species. In particular, a dose of 30 mg/kg seems to be suitable for future studies on developing experimental therapies. These relatively easily induced non-inherited models may serve as useful tools for evaluating novel therapies for RPE-related retinal degenerations, such as AMD.
Keywords: Brown Norway; C57BL/6J; ERG; OCT; macula degeneration; mouse; rat; retinal degeneration; retinal pigment epithelium; rodent; sodium iodate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Course of Sodium Iodate-Induced Retinal Degeneration in Albino and Pigmented Mice.Invest Ophthalmol Vis Sci. 2017 Apr 1;58(4):2239-2249. doi: 10.1167/iovs.16-21255. Invest Ophthalmol Vis Sci. 2017. PMID: 28418497
-
Retinal BMI1 Expression Preserves Photoreceptors in Sodium-Iodate-Induced Oxidative Stress Models.Int J Mol Sci. 2025 Jun 19;26(12):5907. doi: 10.3390/ijms26125907. Int J Mol Sci. 2025. PMID: 40565365 Free PMC article.
-
Sodium Iodate: Rapid and Clinically Relevant Model of AMD.Front Biosci (Landmark Ed). 2024 Nov 13;29(11):380. doi: 10.31083/j.fbl2911380. Front Biosci (Landmark Ed). 2024. PMID: 39614439
-
A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models.Int J Mol Sci. 2020 Apr 14;21(8):2719. doi: 10.3390/ijms21082719. Int J Mol Sci. 2020. PMID: 32295315 Free PMC article.
-
Long time remodeling during retinal degeneration evaluated by optical coherence tomography, immunocytochemistry and fundus autofluorescence.Exp Eye Res. 2016 Sep;150:122-34. doi: 10.1016/j.exer.2015.10.012. Epub 2015 Oct 29. Exp Eye Res. 2016. PMID: 26521765 Review.
Cited by
-
Potential Use of Plasma Rich in Growth Factors in Age-Related Macular Degeneration: Evidence from a Mouse Model.Medicina (Kaunas). 2024 Dec 10;60(12):2036. doi: 10.3390/medicina60122036. Medicina (Kaunas). 2024. PMID: 39768916 Free PMC article.
-
Multi-Wavelength Photobiomodulation Ameliorates Sodium Iodate-Induced Age-Related Macular Degeneration in Rats.Int J Mol Sci. 2023 Dec 12;24(24):17394. doi: 10.3390/ijms242417394. Int J Mol Sci. 2023. PMID: 38139223 Free PMC article.
-
Regulated cell death pathways in the sodium iodate model: Insights and implications for AMD.Exp Eye Res. 2024 Jan;238:109728. doi: 10.1016/j.exer.2023.109728. Epub 2023 Nov 14. Exp Eye Res. 2024. PMID: 37972750 Free PMC article. Review.
-
Retinal Protective Effect of Mono-Ethyl Fumarate in Experimental Age-Related Macular Degeneration via Anti-Oxidative and Anti-Apoptotic Alterations.Int J Mol Sci. 2025 Feb 7;26(4):1413. doi: 10.3390/ijms26041413. Int J Mol Sci. 2025. PMID: 40003880 Free PMC article.
-
Single-cell transcriptome atlas of spontaneous dry age-related macular degeneration in macaques.Fundam Res. 2023 Apr 25;5(3):1034-1046. doi: 10.1016/j.fmre.2023.02.028. eCollection 2025 May. Fundam Res. 2023. PMID: 40528953 Free PMC article.
References
-
- Gorin M.B., Breitner J.C., De Jong P.T., Hageman G.S., Klaver C.C., Kuehn M.H., Seddon J.M. The genetics of age-related macular degeneration. Mol. Vis. 1999;5:29. - PubMed
-
- Klein R., Myers C.E., Buitendijk G.H., Rochtchina E., Gao X., de Jong P.T., Sivakumaran T.A., Burlutsky G., McKean-Cowdin R., Hofman A., et al. Lipids, lipid genes, and incident age-related macular degeneration: The three continent age-related macular degeneration consortium. Am. J. Ophthalmol. 2014;158:513–524.e3. doi: 10.1016/j.ajo.2014.05.027. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

