A Deeper Insight in Metal Binding to the hCtr1 N-terminus Fragment: Affinity, Speciation and Binding Mode of Binuclear Cu2+ and Mononuclear Ag+ Complex Species
- PMID: 35328348
- PMCID: PMC8953729
- DOI: 10.3390/ijms23062929
A Deeper Insight in Metal Binding to the hCtr1 N-terminus Fragment: Affinity, Speciation and Binding Mode of Binuclear Cu2+ and Mononuclear Ag+ Complex Species
Abstract
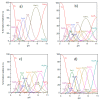
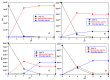
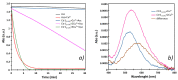
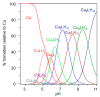
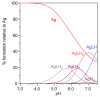
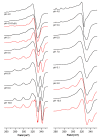
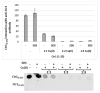
Ctr1 regulates copper uptake and its intracellular distribution. The first 14 amino acid sequence of the Ctr1 ectodomain Ctr1(1-14) encompasses the characteristic Amino Terminal Cu2+ and Ni2+ binding motif (ATCUN) as well as the bis-His binding motif (His5 and His6). We report a combined thermodynamic and spectroscopic (UV-vis, CD, EPR) study dealing with the formation of Cu2+ homobinuclear complexes with Ctr1(1-14), the percentage of which is not negligible even in the presence of a small Cu2+ excess and clearly prevails at a M/L ratio of 1.9. Ascorbate fails to reduce Cu2+ when bound to the ATCUN motif, while it reduces Cu2+ when bound to the His5-His6 motif involved in the formation of binuclear species. The histidine diade characterizes the second binding site and is thought to be responsible for ascorbate oxidation. Binding constants and speciation of Ag+ complexes with Ctr1(1-14), which are assumed to mimic Cu+ interaction with N-terminus of Ctr1(1-14), were also determined. A preliminary immunoblot assay evidences that the anti-Ctr1 extracellular antibody recognizes Ctr1(1-14) in a different way from the longer Ctr1(1-25) that encompasses a second His and Met rich domain.
Keywords: Ctr1; affinity; antibody recognition; copper; model transporter; reductase; silver; speciation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Linder M.C. Biochemistry of Copper. Springer; Boston, MA, USA: 1991. Introduction and Overview of Copper as an Element Essential for Life.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

