The Photoperiod Stress Response in Arabidopsis thaliana Depends on Auxin Acting as an Antagonist to the Protectant Cytokinin
- PMID: 35328357
- PMCID: PMC8955046
- DOI: 10.3390/ijms23062936
The Photoperiod Stress Response in Arabidopsis thaliana Depends on Auxin Acting as an Antagonist to the Protectant Cytokinin
Abstract
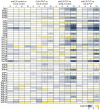
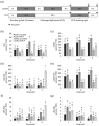
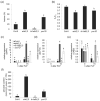
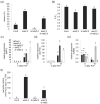
Fluctuating environmental conditions trigger adaptive responses in plants, which are regulated by phytohormones. During photoperiod stress caused by a prolongation of the light period, cytokinin (CK) has a protective function. Auxin often acts as an antagonist of CK in developmental processes and stress responses. Here, we investigated the regulation of the photoperiod stress response in Arabidopsis thaliana by auxin and its interaction with CK. Transcriptome analysis revealed an altered transcript abundance of numerous auxin metabolism and signaling genes after photoperiod stress treatment. The changes appeared earlier and were stronger in the photoperiod-stress-sensitive CK receptor mutant arabidopsis histidine kinase 2 (ahk2),3 compared to wild-type plants. The concentrations of indole-3-acetic acid (IAA), IAA-Glc and IAA-Asp increased in both genotypes, but the increases were more pronounced in ahk2,3. Genetic analysis revealed that the gain-of-function YUCCA 1 (YUC1) mutant, yuc1D, displayed an increased photoperiod stress sensitivity. In contrast, a loss of the auxin receptors TRANSPORT-INHIBITOR-RESISTANT 1 (TIR1), AUXIN SIGNALING F-BOX 2 (AFB2) and AFB3 in wild-type and ahk2,3 background caused a reduced photoperiod stress response. Overall, this study revealed that auxin promotes response to photoperiod stress antagonizing the protective CK.
Keywords: Arabidopsis thaliana; abiotic stress; auxin; crosstalk; cytokinin; photoperiod stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
AUXIN UP-REGULATED F-BOX PROTEIN1 regulates the cross talk between auxin transport and cytokinin signaling during plant root growth.Plant Physiol. 2011 Aug;156(4):1878-93. doi: 10.1104/pp.111.179812. Epub 2011 Jun 8. Plant Physiol. 2011. PMID: 21653785 Free PMC article.
-
Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis.Ann Bot. 2013 Nov;112(7):1395-407. doi: 10.1093/aob/mct215. Epub 2013 Sep 22. Ann Bot. 2013. PMID: 24061489 Free PMC article.
-
Auxin signaling through SCFTIR1/AFBs mediates feedback regulation of IAA biosynthesis.Biosci Biotechnol Biochem. 2017 Jul;81(7):1320-1326. doi: 10.1080/09168451.2017.1313694. Epub 2017 Apr 13. Biosci Biotechnol Biochem. 2017. PMID: 28406060
-
Is auxin enough? Cytokinins and margin patterning in simple leaves.Trends Plant Sci. 2023 Jan;28(1):54-73. doi: 10.1016/j.tplants.2022.08.019. Epub 2022 Sep 27. Trends Plant Sci. 2023. PMID: 36180378 Review.
-
Cytokinin-auxin crosstalk in cell type specification.Trends Plant Sci. 2015 May;20(5):291-300. doi: 10.1016/j.tplants.2015.02.003. Epub 2015 Mar 21. Trends Plant Sci. 2015. PMID: 25805047 Review.
Cited by
-
Perception, Transduction and Crosstalk of Auxin and Cytokinin Signals.Int J Mol Sci. 2022 Oct 29;23(21):13150. doi: 10.3390/ijms232113150. Int J Mol Sci. 2022. PMID: 36361937 Free PMC article.
-
An extragenic second-site mutation in the jar1-1 mutant suppresses the response to photoperiod stress independent of jasmonic acid.Plant Mol Biol. 2025 Jun 29;115(4):79. doi: 10.1007/s11103-025-01602-9. Plant Mol Biol. 2025. PMID: 40581907 Free PMC article.
References
-
- Nitschke S., Cortleven A., Iven T., Feussner I., Havaux M., Riefler M., Schmülling T. Circadian Stress Regimes Affect the Circadian Clock and Cause Jasmonic Acid-Dependent Cell Death in Cytokinin-Deficient Arabidopsis Plants. Plant Cell. 2016;28:1616–1639. doi: 10.1105/tpc.16.00016. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

