The Study of Protein-Cyclitol Interactions
- PMID: 35328362
- PMCID: PMC8952220
- DOI: 10.3390/ijms23062940
The Study of Protein-Cyclitol Interactions
Abstract
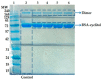
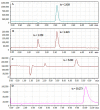
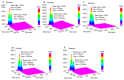
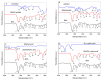
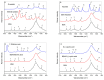
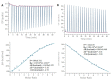
Investigation of interactions between the target protein molecule and ligand allows for an understanding of the nature of the molecular recognition, functions, and biological activity of protein-ligand complexation. In the present work, non-specific interactions between a model protein (Bovine Serum Albumin) and four cyclitols were investigated. D-sorbitol and adonitol represent the group of linear-structure cyclitols, while shikimic acid and D-(-)-quinic acid have cyclic-structure molecules. Various analytical methods, including chromatographic analysis (HPLC-MS/MS), electrophoretic analysis (SDS-PAGE), spectroscopic analysis (spectrofluorimetry, Fourier transform infrared spectroscopy, and Raman spectroscopy), and isothermal titration calorimetry (ITC), were applied for the description of protein-cyclitol interactions. Additionally, computational calculations were performed to predict the possible binding places. Kinetic studies allowed us to clarify interaction mechanisms that may take place during BSA and cyclitol interaction. The results allow us, among other things, to evaluate the impact of the cyclitol's structure on the character of its interactions with the protein.
Keywords: BSA; bonding; cyclitol; interactions; mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Probing the binding of phenolic aldehyde vanillin with bovine serum albumin: Evidence from spectroscopic and docking approach.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Oct 5;203:40-47. doi: 10.1016/j.saa.2018.05.023. Epub 2018 May 8. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29859491
-
Spectroscopic Methodology and Molecular Docking Studies on Changes in Binding Interaction of Felodipine with Bovine Serum Albumin Induced by Cocrystallization with β-Resorcylic Acid.Chem Pharm Bull (Tokyo). 2020;68(10):946-953. doi: 10.1248/cpb.c20-00212. Chem Pharm Bull (Tokyo). 2020. PMID: 32999146
-
Revealing the interaction mechanism between bovine serum albumin (BSA) and a fluorescent coumarin derivative: A multispectroscopic and in silico approach.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Oct 5;318:124466. doi: 10.1016/j.saa.2024.124466. Epub 2024 May 13. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 38761474
-
Combined spectroscopies and molecular docking approach to characterizing the binding interaction of enalapril with bovine serum albumin.Luminescence. 2017 Jun;32(4):481-490. doi: 10.1002/bio.3202. Epub 2016 Aug 23. Luminescence. 2017. PMID: 27550396
-
Molecular binding of toxic phenothiazinium derivatives, azures to bovine serum albumin: A comparative spectroscopic, calorimetric, and in silico study.J Mol Recognit. 2017 Jul;30(7). doi: 10.1002/jmr.2609. Epub 2017 Jan 19. J Mol Recognit. 2017. PMID: 28101950
Cited by
-
Thermodynamic Characterization of the Interaction of Biofunctionalized Gold Nanoclusters with Serum Albumin Using Two- and Three-Dimensional Methods.Int J Mol Sci. 2023 Nov 25;24(23):16760. doi: 10.3390/ijms242316760. Int J Mol Sci. 2023. PMID: 38069083 Free PMC article.
-
Enantioselective Human Serum Albumin Binding of Apremilast: Liquid Chromatographic, Fluorescence and Molecular Docking Study.Int J Mol Sci. 2023 Jan 21;24(3):2168. doi: 10.3390/ijms24032168. Int J Mol Sci. 2023. PMID: 36768492 Free PMC article.
-
Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus.Molecules. 2024 Mar 29;29(7):1528. doi: 10.3390/molecules29071528. Molecules. 2024. PMID: 38611807 Free PMC article.
References
-
- Owczarczyk-Saczonek A., Lahuta L.B., Placek W., Górecki R. The potential benefits of plant cyclitols in the treatment of psoriasis. Pol. Ann. Med. 2018;25:166–171. doi: 10.29089/2017.17.00019. - DOI
-
- Loescher W.H. Physiology and metabolism of sugar alcohols in higher plants. Physiol. Plant. 1987;70:553–557. doi: 10.1111/j.1399-3054.1987.tb02857.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

