A Role of Complement in the Pathogenic Sequelae of Mouse Neonatal Germinal Matrix Hemorrhage
- PMID: 35328364
- PMCID: PMC8954718
- DOI: 10.3390/ijms23062943
A Role of Complement in the Pathogenic Sequelae of Mouse Neonatal Germinal Matrix Hemorrhage
Abstract
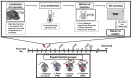
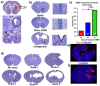
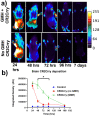
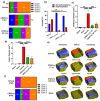
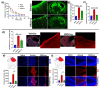
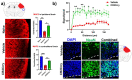
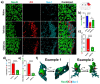
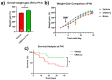
Germinal matrix hemorrhage (GMH) is a devastating disease of infancy that results in intraventricular hemorrhage, post-hemorrhagic hydrocephalus (PHH), periventricular leukomalacia, and neurocognitive deficits. There are no curative treatments and limited surgical options. We developed and characterized a mouse model of GMH based on the injection of collagenase into the subventricular zone of post-natal pups and utilized the model to investigate the role of complement in PHH development. The site-targeted complement inhibitor CR2Crry, which binds deposited C3 complement activation products, localized specifically in the brain following its systemic administration after GMH. Compared to vehicle, CR2Crry treatment reduced PHH and lesion size, which was accompanied by decreased perilesional complement deposition, decreased astrocytosis and microgliosis, and the preservation of dendritic and neuronal density. Complement inhibition also improved survival and weight gain, and it improved motor performance and cognitive outcomes measured in adolescence. The progression to PHH, neuronal loss, and associated behavioral deficits was linked to the microglial phagocytosis of complement opsonized neurons, which was reversed with CR2Crry treatment. Thus, complement plays an important role in the pathological sequelae of GMH, and complement inhibition represents a novel therapeutic approach to reduce the disease progression of a condition for which there is currently no treatment outside of surgical intervention.
Keywords: complement; germinal matrix hemorrhage; hydrocephalus; microglia; neuroinflammation; pediatric.
Conflict of interest statement
S.T. is an inventor of a licensed patent for CR2-targeted complement inhibitors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Design of a Stem Cell-Based Therapy for Ependymal Repair in Hydrocephalus Associated With Germinal Matrix Hemorrhages.Stroke. 2024 Apr;55(4):1062-1074. doi: 10.1161/STROKEAHA.123.044677. Epub 2024 Mar 4. Stroke. 2024. PMID: 38436063 Free PMC article.
-
A role for P-selectin and complement in the pathological sequelae of germinal matrix hemorrhage.J Neuroinflammation. 2023 Jun 16;20(1):143. doi: 10.1186/s12974-023-02828-4. J Neuroinflammation. 2023. PMID: 37322469 Free PMC article.
-
Protective effects of alectinib on germinal matrix hemorrhage-induced neonatal brain injury.Neuroreport. 2025 Aug 6;36(11):599-608. doi: 10.1097/WNR.0000000000002180. Epub 2025 Jun 13. Neuroreport. 2025. PMID: 40530535
-
Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage.Cochrane Database Syst Rev. 2017 Apr 6;4(4):CD000216. doi: 10.1002/14651858.CD000216.pub2. Cochrane Database Syst Rev. 2017. PMID: 28384379 Free PMC article.
-
Head midline position for preventing the occurrence or extension of germinal matrix-intraventricular hemorrhage in preterm infants.Cochrane Database Syst Rev. 2017 Jul 20;7(7):CD012362. doi: 10.1002/14651858.CD012362.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jul 7;7:CD012362. doi: 10.1002/14651858.CD012362.pub3. PMID: 28727900 Free PMC article. Updated.
Cited by
-
Rare variants in complement system genes associate with endothelial damage after pediatric allogeneic hematopoietic stem cell transplantation.Front Immunol. 2023 Sep 13;14:1249958. doi: 10.3389/fimmu.2023.1249958. eCollection 2023. Front Immunol. 2023. PMID: 37771589 Free PMC article.
-
Role of N-formyl peptide receptor 2 in germinal matrix hemorrhage: an intrinsic review of a hematoma resolving pathway.Neural Regen Res. 2024 Feb;19(2):350-354. doi: 10.4103/1673-5374.379040. Neural Regen Res. 2024. PMID: 37488889 Free PMC article. Review.
-
Increased Complement C4 in a Sparse Neuronal Subset Induces Network-Wide Transcriptomic Alterations in the Prefrontal Cortex.bioRxiv [Preprint]. 2025 May 29:2025.05.29.656749. doi: 10.1101/2025.05.29.656749. bioRxiv. 2025. PMID: 40502190 Free PMC article. Preprint.
-
Complement Drives Chronic Inflammation and Progressive Hydrocephalus in Murine Neonatal Germinal Matrix Hemorrhage.Int J Mol Sci. 2023 Jun 15;24(12):10171. doi: 10.3390/ijms241210171. Int J Mol Sci. 2023. PMID: 37373319 Free PMC article.
-
Pharmacological Strategies and Surgical Management of Posthemorrhagic Hydrocephalus Following Germinal Matrix-Intraventricular Hemorrhage in Preterm Infants.Curr Neuropharmacol. 2025;23(3):241-255. doi: 10.2174/1570159X23666240906115817. Curr Neuropharmacol. 2025. PMID: 39248058 Free PMC article. Review.
References
-
- Bolisetty S., Dhawan A., Abdel-Latif M., Bajuk B., Stack J., Oei J.-L., Lui K. New South Wales and Australian Capital Territory Neonatal Intensive Care Units’ Data Collection. Intraventricular Hemorrhage and Neurodevelopmental Outcomes in Extreme Preterm Infants. Pediatrics. 2014;133:55–62. doi: 10.1542/peds.2013-0372. - DOI - PubMed
-
- Radic J.A.E., Vincer M., McNeely P.D. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J. Neurosurg. Pediatr. 2015;15:580–588. doi: 10.3171/2014.11.PEDS14364. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

