Therapeutic Effects of Platelet-Derived Extracellular Vesicles in a Bioengineered Tendon Disease Model
- PMID: 35328370
- PMCID: PMC8954460
- DOI: 10.3390/ijms23062948
Therapeutic Effects of Platelet-Derived Extracellular Vesicles in a Bioengineered Tendon Disease Model
Abstract
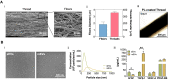
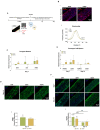
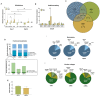
Tendon injuries represent over 30-50% of musculoskeletal disorders worldwide, yet the available therapies do not provide complete tendon repair/regeneration and full functionality restoring. Extracellular vesicles (EVs), membrane-enclosed nanoparticles, have emerged as the next breakthrough in tissue engineering and regenerative medicine to promote endogenous tissue regeneration. Here, we developed a 3D human in vitro model mimicking the signature of pathological tendon and used it to evaluate the influence that different platelet-derived EVs might have in tendon tissue repair mechanisms. For this, different EV populations isolated from platelets, small EVs (sEVs) and medium EVs (mEVs), were added to the culture media of human tendon-derived cells (hTDCs) cultured on isotropic nanofibrous scaffolds. The platelet-derived EVs increased the expression of tenogenic markers, promoted a healthy extracellular matrix (ECM) remodeling, and the synthesis of anti-inflammatory mediators. These findings suggest that platelet EVs provided relevant biochemical cues that potentiated a recovery of hTDCs phenotype from a diseased to a healthy state. Thus, this study opens new perspectives for the translation of platelet-derived EVs as therapeutics.
Keywords: extracellular vesicles; fibers; in vitro models; platelets; tendinopathy; tendon-derived cells; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

