Mechanical-Stress-Related Epigenetic Regulation of ZIC1 Transcription Factor in the Etiology of Postmenopausal Osteoporosis
- PMID: 35328378
- PMCID: PMC8955993
- DOI: 10.3390/ijms23062957
Mechanical-Stress-Related Epigenetic Regulation of ZIC1 Transcription Factor in the Etiology of Postmenopausal Osteoporosis
Abstract
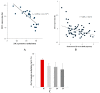
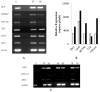
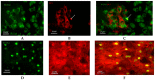
Mechanical loading exerts a profound influence on bone density and architecture, but the exact mechanism is unknown. Our study shows that expression of the neurological transcriptional factor zinc finger of the cerebellum 1 (ZIC1) is markedly increased in trabecular bone biopsies in the lumbar spine compared with the iliac crest, skeletal sites of high and low mechanical stress, respectively. Human trabecular bone transcriptome analyses revealed a strong association between ZIC1 mRNA levels and gene transcripts characteristically associated with osteoblasts, osteocytes and osteoclasts. This supposition is supported by higher ZIC1 expression in iliac bone biopsies from postmenopausal women with osteoporosis compared with age-matched control subjects, as well as strongly significant inverse correlation between ZIC1 mRNA levels and BMI-adjusted bone mineral density (BMD) (Z-score). ZIC1 promoter methylation was decreased in mechanically loaded vertebral bone compared to unloaded normal iliac bone, and its mRNA levels correlated inversely with ZIC1 promoter methylation, thus linking mechanical stress to epigenetic control of gene expression. The findings were corroborated in cultures of rat osteoblast progenitors and osteoblast-like cells. This study demonstrates for the first time how skeletal epigenetic changes that are affected by mechanical forces give rise to marked alteration in bone cell transcriptional activity and translate to human bone pathophysiology.
Keywords: ZIC1 transcription factor; bone; epigenetic; mechanical stress; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Zic1 transcription factor in bone: neural developmental protein regulates mechanotransduction in osteocytes.FASEB J. 2010 Aug;24(8):2893-903. doi: 10.1096/fj.09-148908. Epub 2010 Mar 30. FASEB J. 2010. PMID: 20354137
-
Methylation of bone SOST, its mRNA, and serum sclerostin levels correlate strongly with fracture risk in postmenopausal women.J Bone Miner Res. 2015 Feb;30(2):249-56. doi: 10.1002/jbmr.2342. J Bone Miner Res. 2015. PMID: 25155887
-
Novel Genetic Variants Associated With Increased Vertebral Volumetric BMD, Reduced Vertebral Fracture Risk, and Increased Expression of SLC1A3 and EPHB2.J Bone Miner Res. 2016 Dec;31(12):2085-2097. doi: 10.1002/jbmr.2913. Epub 2016 Sep 6. J Bone Miner Res. 2016. PMID: 27476799 Free PMC article.
-
Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype.J Bone Miner Res. 2011 Aug;26(8):1793-801. doi: 10.1002/jbmr.396. J Bone Miner Res. 2011. PMID: 21452281
-
Aromatase in bone cell: association with osteoporosis in postmenopausal women.J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):165-74. doi: 10.1016/0960-0760(95)00031-t. J Steroid Biochem Mol Biol. 1995. PMID: 7626449 Review.
Cited by
-
Genomic Effects of Biomechanical Loading in Adolescent Human Growth Plate Cartilage: A Pilot Study.Cartilage. 2024 Dec 10:19476035241302954. doi: 10.1177/19476035241302954. Online ahead of print. Cartilage. 2024. PMID: 39655393 Free PMC article.
-
ZIC1 Dictates Osteogenesis Versus Adipogenesis in Human Mesenchymal Progenitor Cells Via a Hedgehog Dependent Mechanism.Stem Cells. 2023 Sep 15;41(9):862-876. doi: 10.1093/stmcls/sxad047. Stem Cells. 2023. PMID: 37317792 Free PMC article.
-
Nat10-mediated N4-acetylcytidine modification enhances Nfatc1 translation to exacerbate osteoclastogenesis in postmenopausal osteoporosis.Proc Natl Acad Sci U S A. 2025 Apr 15;122(15):e2423991122. doi: 10.1073/pnas.2423991122. Epub 2025 Apr 7. Proc Natl Acad Sci U S A. 2025. PMID: 40193598
-
Histone demethylase JMJD3 downregulation protects against aberrant force-induced osteoarthritis through epigenetic control of NR4A1.Int J Oral Sci. 2022 Jul 14;14(1):34. doi: 10.1038/s41368-022-00190-4. Int J Oral Sci. 2022. PMID: 35831280 Free PMC article.
-
The genetic overlap between osteoporosis and craniosynostosis.Front Endocrinol (Lausanne). 2022 Sep 26;13:1020821. doi: 10.3389/fendo.2022.1020821. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36225206 Free PMC article. Review.
References
-
- Kalogeropoulos M., Varanasi S.S., Olstad O.K., Sanderson P., Gautvik V.T., Reppe S., Francis R.M., Gautvik K.M., Birch M.A., Datta H.K. Zic1 transcription factor in bone: Neural developmental protein regulates mechanotransduction in osteocytes. FASEB J. 2010;24:2893–2903. doi: 10.1096/fj.09-148908. - DOI - PubMed
-
- Mansour T.A., Lucot K., Konopelski S.E., Dickinson P.J., Sturges B.K., Vernau K.L., Choi S., Stern J.A., Thomasy S.M., Döring S. Whole genome variant association across 100 dogs identifies a frame shift mutation in DISHEVELLED 2 which contributes to Robinow-like syndrome in Bulldogs and related screw tail dog breeds. PLoS Genet. 2018;14:e1007850. doi: 10.1371/journal.pgen.1007850. - DOI - PMC - PubMed
-
- Medina-Gomez C., Mullin B.H., Chesi A., Prijatelj V., Kemp J.P., Shochat-Carvalho C., Trajanoska K., Wang C., Joro R., Evans T.E. Genome Wide Association Metanalysis of Skull Bone Mineral Density Identifies Loci Relevant for Osteoporosis and Craniosynostosis. medRxiv. 2021 doi: 10.1101/2021.11.01.21265592. - DOI
MeSH terms
Substances
Grants and funding
- MR/R015635/1/MRC_/Medical Research Council/United Kingdom
- # BH121971, EP/K036939/1,Project 29750104,FP6 - 502491/This project was supported by the National Osteoporosis Society (# BH121971), EPSRC (EP/K036939/1), Knowledge Transfer Account from Lovisenberg Diaconal Hospital Oslo, South-Eastern Norway Regional Health Authority (Oslo University Hospital) (Project 2975
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

