The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma
- PMID: 35328425
- PMCID: PMC8949275
- DOI: 10.3390/ijms23063005
The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma
Abstract
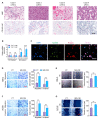
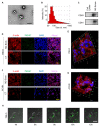
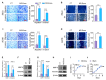
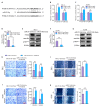
M2 macrophages in the tumor microenvironment are important drivers of cancer metastasis. Exosomes play a critical role in the crosstalk between different cells by delivering microRNAs or other cargos. Whether exosomes derived from pro-tumorigenic M2 macrophages (M2-Exos) could modulate the metastatic behavior of renal cell carcinoma (RCC) is unclear. This study found that M2-Exos promotes migration and invasion in RCC cells. Inhibiting miR-21-5p in M2-Exos significantly reversed their pro-metastatic effects on RCC cells in vitro and in the avian embryo chorioallantoic membrane in vivo tumor model. We further found that the pro-metastatic mechanism of miR-21-5p in M2-Exos is by targeting PTEN-3'UTR to regulate PTEN/Akt signaling. Taken together, our results demonstrate that M2-Exos carries miR-21-5p promote metastatic features of RCC cells through PTEN/Akt signaling. Reversing this could serve as a novel approach to control RCC metastasis.
Keywords: M2 macrophages; PTEN/Akt; exosomes; invasion; miRNA-21-5p; migration; renal cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

