Intracavernous Injection of Platelet-Rich Plasma Therapy Enhances Erectile Function and Decreases the Mortality Rate in Streptozotocin-Induced Diabetic Rats
- PMID: 35328437
- PMCID: PMC8948834
- DOI: 10.3390/ijms23063017
Intracavernous Injection of Platelet-Rich Plasma Therapy Enhances Erectile Function and Decreases the Mortality Rate in Streptozotocin-Induced Diabetic Rats
Abstract
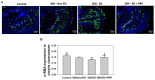
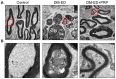
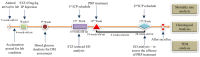
Erectile dysfunction (ED) is an agonizing complication of diabetes mellitus (DM) and it is challenging to treat ED in DM patients. Platelet-rich plasma (PRP) is a unique therapeutic strategy comprising intrinsic growth factors. An attempt was made to explore the potentiality of the PRP treatment in DM-induced ED rats in various groups (control, DM-non-ED, DM-ED, and DM-ED treated with PRP). Streptozotocin (STZ) was used to induce DM in rats. The blood glucose levels of the DM rats were maintained at >300 mg/dl. In the 18-week experiment, survival rate, body weight, intracavernous pressure (ICP) variations, and arterial blood pressure were analyzed. The tissue restoration results were validated by histological, immunofluorescence, and transmission electron microscopic analysis. PRP treatment of DM-ED rats significantly increased all parameters of erectile function compared to pre-treatment of PRP and DM-ED treated with vehicle. The histological results revealed that PRP treatment substantially enhanced the regeneration of myelinated nerves and decreased the atrophy of corporal smooth muscle. Notably, the PRP treatment immensely enhanced the survival rate in post-surgery DM-ED rats. These results indicated certain benefits of PRP treatment in delaying damage and preventing post-surgery complications in DM patients. Hence, PRP treatment is a novel multifactorial strategy for DM-ED patients.
Keywords: diabetes mellitus; erectile dysfunction; neuroregeneration; platelet-rich plasma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Intracavernous injection of platelet-rich plasma reverses erectile dysfunction of chronic cavernous nerve degeneration through reduction of prostate hyperplasia evidence from an aging-induced erectile dysfunction rat model.FASEB J. 2023 Apr;37(4):e22826. doi: 10.1096/fj.202201443R. FASEB J. 2023. PMID: 36856608 Free PMC article.
-
Effects of platelet-rich plasma glue placement at the prostatectomy site on erectile function restoration and cavernous nerve preservation in a nerve-sparing prostatectomy rat model.Biomed Pharmacother. 2023 May;161:114499. doi: 10.1016/j.biopha.2023.114499. Epub 2023 Mar 11. Biomed Pharmacother. 2023. PMID: 36913891
-
Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats.J Sex Med. 2011 Feb;8(2):427-36. doi: 10.1111/j.1743-6109.2010.02118.x. Epub 2010 Nov 22. J Sex Med. 2011. PMID: 21091881
-
Platelet-Rich Plasma Therapy for Male Sexual Dysfunction: Myth or Reality?Sex Med Rev. 2020 Jan;8(1):106-113. doi: 10.1016/j.sxmr.2019.02.002. Epub 2019 Mar 19. Sex Med Rev. 2020. PMID: 30898594 Review.
-
Platelet-rich plasma for erectile dysfunction: a review of the current research landscape.Sex Med Rev. 2023 Sep 27;11(4):369-374. doi: 10.1093/sxmrev/qead032. Sex Med Rev. 2023. PMID: 37786350 Review.
Cited by
-
Standardization of Animal Models and Techniques for Platelet-Rich Fibrin Production: A Narrative Review and Guideline.Bioengineering (Basel). 2023 Apr 17;10(4):482. doi: 10.3390/bioengineering10040482. Bioengineering (Basel). 2023. PMID: 37106669 Free PMC article. Review.
-
Platelet-rich plasma (PRP) in nerve repair.Regen Ther. 2024 Apr 4;27:244-250. doi: 10.1016/j.reth.2024.03.017. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38586873 Free PMC article. Review.
-
Erectile dysfunction: Is platelet-rich plasma the new frontier for treatment in patients with erectile dysfunction? A review of the existing evidence.Front Reprod Health. 2022 Aug 16;4:944765. doi: 10.3389/frph.2022.944765. eCollection 2022. Front Reprod Health. 2022. PMID: 36303622 Free PMC article. Review.
-
Repeated platelet-rich plasma injections improve erectile dysfunction in a rat model of hyperhomocysteinemia.Asian J Androl. 2024 Nov 1;26(6):622-627. doi: 10.4103/aja202418. Epub 2024 Jul 2. Asian J Androl. 2024. PMID: 38953713 Free PMC article.
-
Intracavernous injection of platelet-rich plasma reverses erectile dysfunction of chronic cavernous nerve degeneration through reduction of prostate hyperplasia evidence from an aging-induced erectile dysfunction rat model.FASEB J. 2023 Apr;37(4):e22826. doi: 10.1096/fj.202201443R. FASEB J. 2023. PMID: 36856608 Free PMC article.
References
-
- Schauer I., Keller E., Müller A., Madersbacher S. Have rates of erectile dysfunction improved within the past 17 years after radical prostatectomy? A systematic analysis of the control arms of prospective randomized trials on penile rehabilitation. Andrology. 2015;3:661–665. doi: 10.1111/andr.12060. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- MOST108-2320-B-030-006/Ministry of Science and Technology
- 105-CM-FJU-03, 105-CM-FJU-01/Chi Mei Medical Center and Fu Jen Catholic University Education and Development Cooperation Project
- 109-CGH-FJU-07/Cathay General Hospital and Fu Jen Catholic University Education and Development Cooperation Project
- 110-FEMH-FJU-03/Far Eastern Memorial Hospital and Fu Jen Catholic University Education and Development Cooperation Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials

