Tuning Strain Stiffening of Protein Hydrogels by Charge Modification
- PMID: 35328457
- PMCID: PMC8950043
- DOI: 10.3390/ijms23063032
Tuning Strain Stiffening of Protein Hydrogels by Charge Modification
Abstract
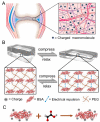
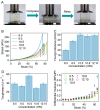
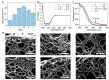
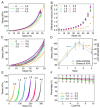
Strain-stiffening properties derived from biological tissue have been widely observed in biological hydrogels and are essential in mimicking natural tissues. Although strain-stiffening has been studied in various protein-based hydrogels, effective approaches for tuning the strain-stiffening properties of protein hydrogels have rarely been explored. Here, we demonstrated a new method to tune the strain-stiffening amplitudes of protein hydrogels. By adjusting the surface charge of proteins inside the hydrogel using negatively/positively charged molecules, the strain-stiffening amplitudes could be quantitively regulated. The strain-stiffening of the protein hydrogels could even be enhanced 5-fold under high deformations, while the bulk property, recovery ability and biocompatibility remained almost unchanged. The tuning of strain-stiffening amplitudes using different molecules or in different protein hydrogels was further proved to be feasible. We anticipate that surface charge adjustment of proteins in hydrogels represents a general principle to tune the strain-stiffening property and can find wide applications in regulating the mechanical behaviors of protein-based hydrogels.
Keywords: electrical repulsion; mechanical property; protein hydrogel; strain-stiffening; surface charge.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Humphrey J.D. Continuum biomechanics of soft biological tissues. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2003;459:3–46. doi: 10.1098/rspa.2002.1060. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

