Single-Cell RNA Sequencing with Spatial Transcriptomics of Cancer Tissues
- PMID: 35328458
- PMCID: PMC8955933
- DOI: 10.3390/ijms23063042
Single-Cell RNA Sequencing with Spatial Transcriptomics of Cancer Tissues
Abstract
Single-cell RNA sequencing (RNA-seq) techniques can perform analysis of transcriptome at the single-cell level and possess an unprecedented potential for exploring signatures involved in tumor development and progression. These techniques can perform sequence analysis of transcripts with a better resolution that could increase understanding of the cellular diversity found in the tumor microenvironment and how the cells interact with each other in complex heterogeneous cancerous tissues. Identifying the changes occurring in the genome and transcriptome in the spatial context is considered to increase knowledge of molecular factors fueling cancers. It may help develop better monitoring strategies and innovative approaches for cancer treatment. Recently, there has been a growing trend in the integration of RNA-seq techniques with contemporary omics technologies to study the tumor microenvironment. There has been a realization that this area of research has a huge scope of application in translational research. This review article presents an overview of various types of single-cell RNA-seq techniques used currently for analysis of cancer tissues, their pros and cons in bulk profiling of transcriptome, and recent advances in the techniques in exploring heterogeneity of various types of cancer tissues. Furthermore, we have highlighted the integration of single-cell RNA-seq techniques with other omics technologies for analysis of transcriptome in their spatial context, which is considered to revolutionize the understanding of tumor microenvironment.
Keywords: intratumor heterogeneity; single-cell RNA sequencing techniques; spatial transcriptomics.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
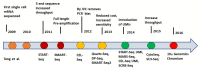
Figures



References
-
- Heng H.H., Stevens J.B., Bremer S.W., Liu G., Abdallah B.Y., Christine J.Y. Evolutionary mechanisms and diversity in cancer. Adv. Cancer Res. 2011;112:217–253. - PubMed
-
- Saltz J., Gupta R., Hou L., Kurc T., Singh P., Nguyen V., Samaras D., Shroyer K.R., Zhao T., Batiste R. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 2018;23:181–193.e7. doi: 10.1016/j.celrep.2018.03.086. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

