Chiral Linked Systems as a Model for Understanding D-Amino Acids Influence on the Structure and Properties of Amyloid Peptides
- PMID: 35328481
- PMCID: PMC8955658
- DOI: 10.3390/ijms23063060
Chiral Linked Systems as a Model for Understanding D-Amino Acids Influence on the Structure and Properties of Amyloid Peptides
Abstract
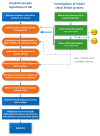
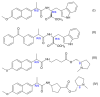
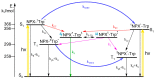
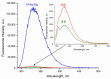
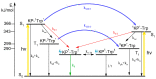
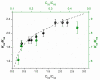
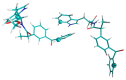
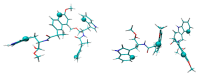
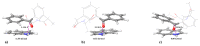
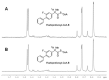
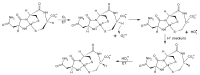
In this review, we provide an illustration of the idea discussed in the literature of using model compounds to study the effect of substitution of L- for D-amino acid residues in amyloid peptides. The need for modeling is due to the inability to study highly disordered peptides by traditional methods (high-field NMR, X-ray). At the same time, the appearance of such peptides, where L-amino acids are partially replaced by D-analogs is one of the main causes of Alzheimer's disease. The review presents examples of the use diastereomers with L-/D-tryptophan in model process-photoinduced electron transfer (ET) for studying differences in reactivity and structure of systems with L- and D-optical isomers. The combined application of spin effects, including those calculated using the original theory, fluorescence techniques and molecular modeling has demonstrated a real difference in the structure and efficiency of ET in diastereomers with L-/D-tryptophan residues. In addition, the review compared the factors governing chiral inversion in model metallopeptides and Aβ42 amyloid.
Keywords: D-amino acids; amyloid peptides; chiral inversion; chiral linked systems; diastereomers; electron transfer; fluorescence quenching; molecular dynamics; spin effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
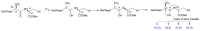
References
-
- Tverdislov V.A., Yakovenko L.V., Zhavoronkov A.A. Chirality as a problem of biochemical physics. Russ. J. Gen. Chem. 2007;77:1994–2005. doi: 10.1134/S1070363207110291. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

