Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers
- PMID: 35328483
- PMCID: PMC8949414
- DOI: 10.3390/ijms23063062
Gene-Delivery Ability of New Hydrogenated and Partially Fluorinated Gemini bispyridinium Surfactants with Six Methylene Spacers
Abstract
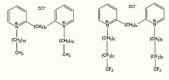
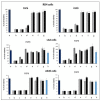
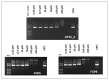
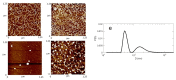
The pandemic emergency determined by the spreading worldwide of the SARS-CoV-2 virus has focused the scientific and economic efforts of the pharmaceutical industry and governments on the possibility to fight the virus by genetic immunization. The genetic material must be delivered inside the cells by means of vectors. Due to the risk of adverse or immunogenic reaction or replication connected with the more efficient viral vectors, non-viral vectors are in many cases considered as a preferred strategy for gene delivery into eukaryotic cells. This paper is devoted to the evaluation of the gene delivery ability of new synthesized gemini bis-pyridinium surfactants with six methylene spacers, both hydrogenated and fluorinated, in comparison with compounds with spacers of different lengths, previously studied. Results from MTT proliferation assay, electrophoresis mobility shift assay (EMSA), transient transfection assay tests and atomic force microscopy (AFM) imaging confirm that pyridinium gemini surfactants could be a valuable tool for gene delivery purposes, but their performance is highly dependent on the spacer length and strictly related to their structure in solution. All the fluorinated compounds are unable to transfect RD-4 cells, if used alone, but they are all able to deliver a plasmid carrying an enhanced green fluorescent protein (EGFP) expression cassette, when co-formulated with 1,2-dioleyl-sn-glycero-3-phosphoethanolamine (DOPE) in a 1:2 ratio. The fluorinated compounds with spacers formed by six (FGP6) and eight carbon atoms (FGP8) give rise to a very interesting gene delivery activity, greater to that of the commercial reagent, when formulated with DOPE. The hydrogenated compound GP16_6 is unable to sufficiently compact the DNA, as shown by AFM images.
Keywords: DNA-surfactant interaction; atomic force microscopy on DNA; gene delivery; gene therapy; heterocyclic gemini cationic surfactants; nonviral vectors; partially fluorinated gemini surfactants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chemico-Physical Properties of Some 1,1'-Bis-alkyl-2,2'-hexane-1,6-diyl-bispyridinium Chlorides Hydrogenated and Partially Fluorinated for Gene Delivery.Molecules. 2023 Apr 20;28(8):3585. doi: 10.3390/molecules28083585. Molecules. 2023. PMID: 37110819 Free PMC article.
-
Nonviral gene-delivery by highly fluorinated gemini bispyridinium surfactant-based DNA nanoparticles.J Colloid Interface Sci. 2017 Feb 1;487:182-191. doi: 10.1016/j.jcis.2016.10.032. Epub 2016 Oct 15. J Colloid Interface Sci. 2017. PMID: 27769002
-
Nonviral gene delivery: gemini bispyridinium surfactant-based DNA nanoparticles.J Phys Chem B. 2014 Nov 20;118(46):13183-91. doi: 10.1021/jp507999g. Epub 2014 Nov 10. J Phys Chem B. 2014. PMID: 25340646
-
Interactions between DNA and gemini surfactant: impact on gene therapy: part II.Nanomedicine (Lond). 2016 Feb;11(4):403-20. doi: 10.2217/nnm.15.204. Epub 2016 Jan 19. Nanomedicine (Lond). 2016. PMID: 26784450 Review.
-
Interactions between DNA and Gemini surfactant: impact on gene therapy: part I.Nanomedicine (Lond). 2016 Feb;11(3):289-306. doi: 10.2217/nnm.15.203. Epub 2016 Jan 20. Nanomedicine (Lond). 2016. PMID: 26785905 Review.
Cited by
-
Chemico-Physical Properties of Some 1,1'-Bis-alkyl-2,2'-hexane-1,6-diyl-bispyridinium Chlorides Hydrogenated and Partially Fluorinated for Gene Delivery.Molecules. 2023 Apr 20;28(8):3585. doi: 10.3390/molecules28083585. Molecules. 2023. PMID: 37110819 Free PMC article.
-
Development of Self-Assembling bis-1,4-Dihydropyridines: Detailed Studies of Bromination of Four Methyl Groups and Bromine Nucleophilic Substitution.Molecules. 2023 Dec 27;29(1):161. doi: 10.3390/molecules29010161. Molecules. 2023. PMID: 38202746 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

