Vasopressin and Its Analogues: From Natural Hormones to Multitasking Peptides
- PMID: 35328489
- PMCID: PMC8955888
- DOI: 10.3390/ijms23063068
Vasopressin and Its Analogues: From Natural Hormones to Multitasking Peptides
Abstract
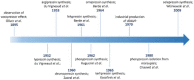
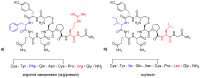
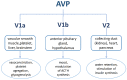
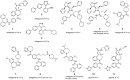
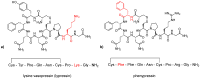
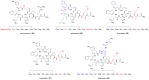
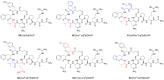
Human neurohormone vasopressin (AVP) is synthesized in overlapping regions in the hypothalamus. It is mainly known for its vasoconstricting abilities, and it is responsible for the regulation of plasma osmolality by maintaining fluid homeostasis. Over years, many attempts have been made to modify this hormone and find AVP analogues with different pharmacological profiles that could overcome its limitations. Non-peptide AVP analogues with low molecular weight presented good affinity to AVP receptors. Natural peptide counterparts, found in animals, are successfully applied as therapeutics; for instance, lypressin used in treatment of diabetes insipidus. Synthetic peptide analogues compensate for the shortcomings of AVP. Desmopressin is more resistant to proteolysis and presents mainly antidiuretic effects, while terlipressin is a long-acting AVP analogue and a drug recommended in the treatment of varicose bleeding in patients with liver cirrhosis. Recently published results on diverse applications of AVP analogues in medicinal practice, including potential lypressin, terlipressin and ornipressin in the treatment of SARS-CoV-2, are discussed.
Keywords: desmopressin; vasoconstrictors; vasopressin; vasopressin analogues; vasopressin receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Grauer A., König B. Peptidomimetics—A versatile route to biologically active compounds. Eur. J. Org. Chem. 2009;30:5099–5111. doi: 10.1002/ejoc.200900599. - DOI
-
- Vaisman A., Lushchak O. Encyclopedia of Biomedical Gerontology, Reference Modul in Biomedical Science. 1st ed. Elsevier Inc. Academic Press; Aalborg, Denmark: 2020. Geroscience; pp. 154–159. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

