Neuroinflammation and COVID-19 Ischemic Stroke Recovery-Evolving Evidence for the Mediating Roles of the ACE2/Angiotensin-(1-7)/Mas Receptor Axis and NLRP3 Inflammasome
- PMID: 35328506
- PMCID: PMC8949282
- DOI: 10.3390/ijms23063085
Neuroinflammation and COVID-19 Ischemic Stroke Recovery-Evolving Evidence for the Mediating Roles of the ACE2/Angiotensin-(1-7)/Mas Receptor Axis and NLRP3 Inflammasome
Abstract
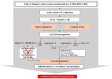
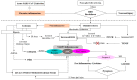
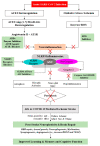
Cerebrovascular events, notably acute ischemic strokes (AIS), have been reported in the setting of novel coronavirus disease (COVID-19) infection. Commonly regarded as cryptogenic, to date, the etiology is thought to be multifactorial and remains obscure; it is linked either to a direct viral invasion or to an indirect virus-induced prothrombotic state, with or without the presence of conventional cerebrovascular risk factors. In addition, patients are at a greater risk of developing long-term negative sequelae, i.e., long-COVID-related neurological problems, when compared to non-COVID-19 stroke patients. Central to the underlying neurobiology of stroke recovery in the context of COVID-19 infection is reduced angiotensin-converting enzyme 2 (ACE2) expression, which is known to lead to thrombo-inflammation and ACE2/angiotensin-(1-7)/mitochondrial assembly receptor (MasR) (ACE2/Ang-(1-7)/MasR) axis inhibition. Moreover, after AIS, the activated nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome may heighten the production of numerous proinflammatory cytokines, mediating neuro-glial cell dysfunction, ultimately leading to nerve-cell death. Therefore, potential neuroprotective therapies targeting the molecular mechanisms of the aforementioned mediators may help to inform rehabilitation strategies to improve brain reorganization (i.e., neuro-gliogenesis and synaptogenesis) and secondary prevention among AIS patients with or without COVID-19. Therefore, this narrative review aims to evaluate the mediating role of the ACE2/Ang- (1-7)/MasR axis and NLRP3 inflammasome in COVID-19-mediated AIS, as well as the prospects of these neuroinflammation mediators for brain repair and in secondary prevention strategies against AIS in stroke rehabilitation.
Keywords: ACE2; COVID-19; NLRP3 inflammasome; ischemic stroke; neurorehabilitation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Involvement of the ACE2/Ang-(1-7)/MasR Axis in Pulmonary Fibrosis: Implications for COVID-19.Int J Mol Sci. 2021 Nov 30;22(23):12955. doi: 10.3390/ijms222312955. Int J Mol Sci. 2021. PMID: 34884756 Free PMC article. Review.
-
Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke.J Neuroinflammation. 2021 May 31;18(1):123. doi: 10.1186/s12974-021-02137-8. J Neuroinflammation. 2021. PMID: 34059091 Free PMC article. Review.
-
Janus Kinase Inhibition Ameliorates Ischemic Stroke Injury and Neuroinflammation Through Reducing NLRP3 Inflammasome Activation via JAK2/STAT3 Pathway Inhibition.Front Immunol. 2021 Jul 22;12:714943. doi: 10.3389/fimmu.2021.714943. eCollection 2021. Front Immunol. 2021. PMID: 34367186 Free PMC article.
-
The angiotensin-converting enzyme 2/angiotensin (1-7)/mas axis protects against pyroptosis in LPS-induced lung injury by inhibiting NLRP3 activation.Arch Biochem Biophys. 2020 Oct 30;693:108562. doi: 10.1016/j.abb.2020.108562. Epub 2020 Aug 28. Arch Biochem Biophys. 2020. PMID: 32866470
-
Activation of angiotensin-converting enzyme 2/angiotensin (1-7)/mas receptor axis triggers autophagy and suppresses microglia proinflammatory polarization via forkhead box class O1 signaling.Aging Cell. 2021 Oct;20(10):e13480. doi: 10.1111/acel.13480. Epub 2021 Sep 16. Aging Cell. 2021. PMID: 34529881 Free PMC article.
Cited by
-
COVID-19 and atrial fibrillation: Intercepting lines.Front Cardiovasc Med. 2023 Jan 23;10:1093053. doi: 10.3389/fcvm.2023.1093053. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36755799 Free PMC article. Review.
-
Intersecting Pathways: The Role of Metabolic Dysregulation, Gastrointestinal Microbiome, and Inflammation in Acute Ischemic Stroke Pathogenesis and Outcomes.J Clin Med. 2024 Jul 21;13(14):4258. doi: 10.3390/jcm13144258. J Clin Med. 2024. PMID: 39064298 Free PMC article. Review.
-
Prescription of selective serotonin reuptake inhibitors in COVID-19 infection needs caution.Front Psychiatry. 2022 Oct 19;13:1052710. doi: 10.3389/fpsyt.2022.1052710. eCollection 2022. Front Psychiatry. 2022. PMID: 36339865 Free PMC article. No abstract available.
-
The NLRP3 Inflammasome in Age-Related Cerebral Small Vessel Disease Manifestations: Untying the Innate Immune Response Connection.Life (Basel). 2023 Jan 12;13(1):216. doi: 10.3390/life13010216. Life (Basel). 2023. PMID: 36676165 Free PMC article. Review.
-
Angiotensin-(1-7) Provides Potent Long-Term Neurorepair/Neuroregeneration in a Rodent White Matter Stroke Model: Nonarteritic Ischemic Optic Neuropathy (rNAION).Cells. 2025 Feb 15;14(4):289. doi: 10.3390/cells14040289. Cells. 2025. PMID: 39996759 Free PMC article.
References
-
- Najjar S., Najjar A., Chong D.J., Pramanik B.K., Kirsch C., Kuzniecky R.I., Pacia S.V., Azhar S. Central nervous system complications associated with SARS-CoV-2 infection: Integrative concepts of pathophysiology and case reports. J. Neuroinflamm. 2020;17:231. doi: 10.1186/s12974-020-01896-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

