R-Spondin2, a Positive Canonical WNT Signaling Regulator, Controls the Expansion and Differentiation of Distal Lung Epithelial Stem/Progenitor Cells in Mice
- PMID: 35328508
- PMCID: PMC8954098
- DOI: 10.3390/ijms23063089
R-Spondin2, a Positive Canonical WNT Signaling Regulator, Controls the Expansion and Differentiation of Distal Lung Epithelial Stem/Progenitor Cells in Mice
Abstract
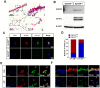
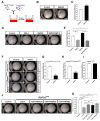
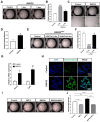
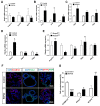
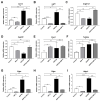
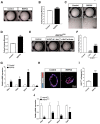
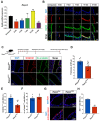
The lungs have a remarkable ability to regenerate damaged tissues caused by acute injury. Many lung diseases, especially chronic lung diseases, are associated with a reduced or disrupted regeneration potential of the lungs. Therefore, understanding the underlying mechanisms of the regenerative capacity of the lungs offers the potential to identify novel therapeutic targets for these diseases. R-spondin2, a co-activator of WNT/β-catenin signaling, plays an important role in embryonic murine lung development. However, the role of Rspo2 in adult lung homeostasis and regeneration remains unknown. The aim of this study is to determine Rspo2 function in distal lung stem/progenitor cells and adult lung regeneration. In this study, we found that robust Rspo2 expression was detected in different epithelial cells, including airway club cells and alveolar type 2 (AT2) cells in the adult lungs. However, Rspo2 expression significantly decreased during the first week after naphthalene-induced airway injury and was restored by day 14 post-injury. In ex vivo 3D organoid culture, recombinant RSPO2 promoted the colony formation and differentiation of both club and AT2 cells through the activation of canonical WNT signaling. In contrast, Rspo2 ablation in club and AT2 cells significantly disrupted their expansion capacity in the ex vivo 3D organoid culture. Furthermore, mice lacking Rspo2 showed significant defects in airway regeneration after naphthalene-induced injury. Our results strongly suggest that RSPO2 plays a key role in the adult lung epithelial stem/progenitor cells during homeostasis and regeneration, and therefore, it may be a potential therapeutic target for chronic lung diseases with reduced regenerative capability.
Keywords: AT2 cells; R-spondin; RSPO2; WNT signaling; club cells; lung homeostasis; lung regeneration; lung stem/progenitor cells; β-catenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema.Stem Cells. 2020 Nov;38(11):1467-1478. doi: 10.1002/stem.3241. Epub 2020 Jun 18. Stem Cells. 2020. PMID: 32526076 Free PMC article.
-
R-Spondin 2 Induces Odontogenic Differentiation of Dental Pulp Stem/Progenitor Cells via Regulation of Wnt/β-Catenin Signaling.Front Physiol. 2020 Aug 7;11:918. doi: 10.3389/fphys.2020.00918. eCollection 2020. Front Physiol. 2020. PMID: 32848860 Free PMC article.
-
R-spondin 2 facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes by enhancing Wnt/β-catenin signaling in endochondral ossification.Biochem Biophys Res Commun. 2016 Apr 22;473(1):255-264. doi: 10.1016/j.bbrc.2016.03.089. Epub 2016 Mar 22. Biochem Biophys Res Commun. 2016. PMID: 27012200
-
The roles of Wnt/β-catenin pathway in tissue development and regenerative medicine.J Cell Physiol. 2018 Aug;233(8):5598-5612. doi: 10.1002/jcp.26265. Epub 2018 Mar 7. J Cell Physiol. 2018. PMID: 29150936 Review.
-
WNT Signaling in Lung Repair and Regeneration.Mol Cells. 2020 Sep 30;43(9):774-783. doi: 10.14348/molcells.2020.0059. Mol Cells. 2020. PMID: 32807748 Free PMC article. Review.
Cited by
-
Alveolar Type 2 Epithelial Cell Organoids: Focus on Culture Methods.Biomedicines. 2023 Nov 12;11(11):3034. doi: 10.3390/biomedicines11113034. Biomedicines. 2023. PMID: 38002035 Free PMC article. Review.
-
[Role of R-spondin 2 on osteogenic differentiation of bone marrow mesenchymal stem cells and bone metabolism in ovariectomized mice].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Nov 15;38(11):1399-1407. doi: 10.7507/1002-1892.202406083. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 39542634 Free PMC article. Chinese.
-
The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients.Int J Mol Sci. 2023 Sep 5;24(18):13669. doi: 10.3390/ijms241813669. Int J Mol Sci. 2023. PMID: 37761972 Free PMC article.
-
The biological functions and related signaling pathways of SPON2.Front Oncol. 2024 Jan 9;13:1323744. doi: 10.3389/fonc.2023.1323744. eCollection 2023. Front Oncol. 2024. PMID: 38264743 Free PMC article. Review.
-
A gene edited pig model for studying LGR5+ stem cells: implications for future applications in tissue regeneration and biomedical research.Front Genome Ed. 2024 Jun 6;6:1401163. doi: 10.3389/fgeed.2024.1401163. eCollection 2024. Front Genome Ed. 2024. PMID: 38903529 Free PMC article. Review.
References
-
- Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C., Niklason L., Calle E., Le A., Randell S.H., et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. - DOI - PMC - PubMed
-
- Lynch T.J., Anderson P.J., Rotti P.G., Tyler S.R., Crooke A.K., Choi S.H., Montoro D.T., Silverman C.L., Shahin W., Zhao R., et al. Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell. 2018;22:653–667.E5. doi: 10.1016/j.stem.2018.03.017. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

