Detection of Human Neutrophil Elastase by Fluorescent Peptide Sensors Conjugated to TEMPO-Oxidized Nanofibrillated Cellulose
- PMID: 35328520
- PMCID: PMC8952216
- DOI: 10.3390/ijms23063101
Detection of Human Neutrophil Elastase by Fluorescent Peptide Sensors Conjugated to TEMPO-Oxidized Nanofibrillated Cellulose
Abstract
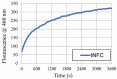
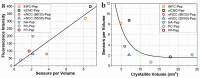
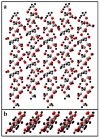
Peptide-cellulose conjugates designed for use as optical protease sensors have gained interest for point-of-care (POC) detection. Elevated serine protease levels are often found in patients with chronic illnesses, necessitating optimal biosensor design for POC assessment. Nanocellulose provides a platform for protease sensors as a transducer surface, and the employment of nanocellulose in this capacity combines its biocompatibility and high specific surface area properties to confer sensitive detection of dilute biomarkers. However, a basic understanding of the spatiotemporal relationships of the transducer surface and sensor disposition is needed to improve protease sensor design and development. Here, we examine a tripeptide, fluorogenic elastase biosensor attached to TEMPO-oxidized nanofibrillated cellulose via a polyethylene glycol linker. The synthetic conjugate was found to be active in the presence of human neutrophil elastase at levels comparable to other cellulose-based biosensors. Computational models examined the relationship of the sensor molecule to the transducer surface. The results illustrate differences in two crystallite transducer surfaces ((110) vs. (1-10)) and reveal preferred orientations of the sensor. Finally, a determination of the relative (110) vs. (1-10) orientations of crystals extracted from cotton demonstrates a preference for the (1-10) conformer. This model study potentiates the HNE sensor results for enhanced sensor activity design.
Keywords: TEMPO-oxidized nanofibrillated cellulose; biosensors; computational modeling; human neutrophil elastase; peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jorfi M., Foster E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015;132:41719. doi: 10.1002/app.41719. - DOI
-
- Frazier T., Alarcon A., Wu X., Mohiuddin O.A., Motherwell J.M., Carlsson A.H., Christy R.J., Edwards J.V., Mackin R.T., Prevost N., et al. Clinical Translational Potential in Skin Wound Regeneration for Adipose-Derived, Blood-Derived, and Cellulose Materials: Cells, Exosomes, and Hydrogels. Biomolecules. 2020;10:1373. doi: 10.3390/biom10101373. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

